The Four Emetic Risk Groups HIGH Risk in














- Slides: 30






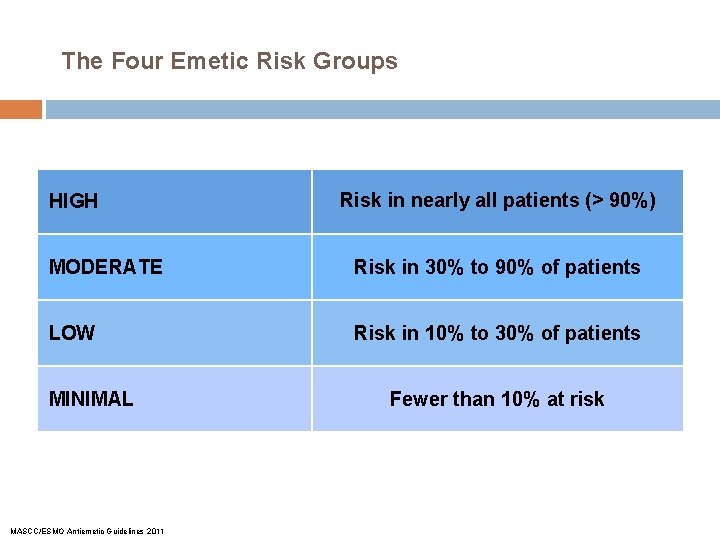
The Four Emetic Risk Groups HIGH Risk in nearly all patients (> 90%) MODERATE Risk in 30% to 90% of patients LOW Risk in 10% to 30% of patients MINIMAL MASCC/ESMO Antiemetic Guidelines 2011 Fewer than 10% at risk

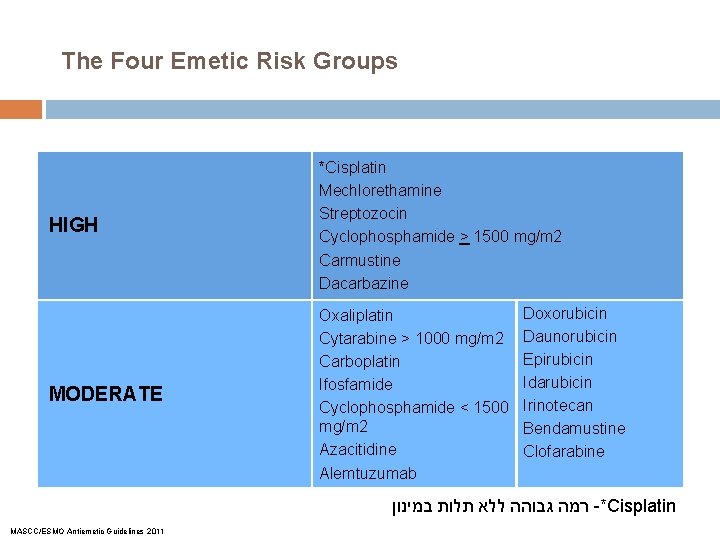
The Four Emetic Risk Groups HIGH *Cisplatin Mechlorethamine Streptozocin Cyclophosphamide > 1500 mg/m 2 Carmustine Dacarbazine MODERATE Oxaliplatin Cytarabine > 1000 mg/m 2 Carboplatin Ifosfamide Cyclophosphamide < 1500 mg/m 2 Azacitidine Alemtuzumab Doxorubicin Daunorubicin Epirubicin Idarubicin Irinotecan Bendamustine Clofarabine רמה גבוהה ללא תלות במינון -*Cisplatin MASCC/ESMO Antiemetic Guidelines 2011

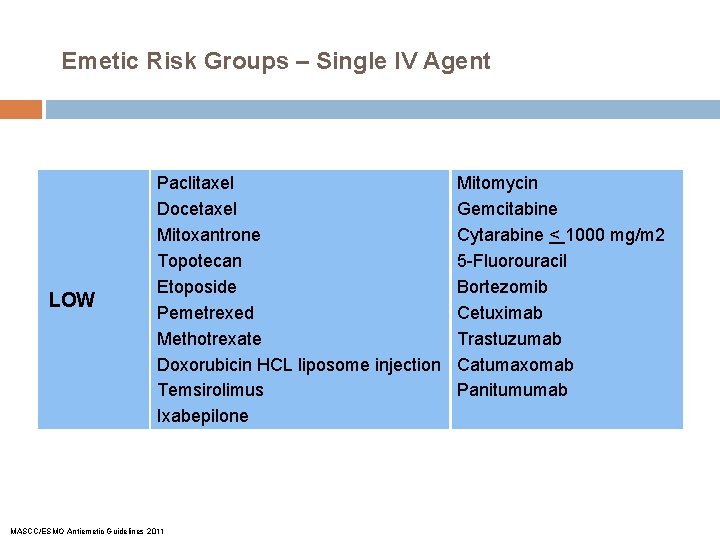
Emetic Risk Groups – Single IV Agent LOW Paclitaxel Docetaxel Mitoxantrone Topotecan Etoposide Pemetrexed Methotrexate Doxorubicin HCL liposome injection Temsirolimus Ixabepilone MASCC/ESMO Antiemetic Guidelines 2011 Mitomycin Gemcitabine Cytarabine < 1000 mg/m 2 5 -Fluorouracil Bortezomib Cetuximab Trastuzumab Catumaxomab Panitumumab

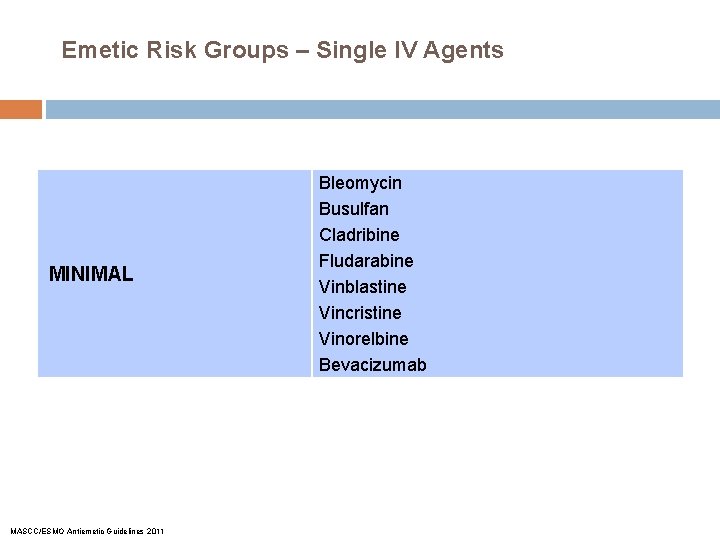
Emetic Risk Groups – Single IV Agents MINIMAL MASCC/ESMO Antiemetic Guidelines 2011 Bleomycin Busulfan Cladribine Fludarabine Vinblastine Vincristine Vinorelbine Bevacizumab

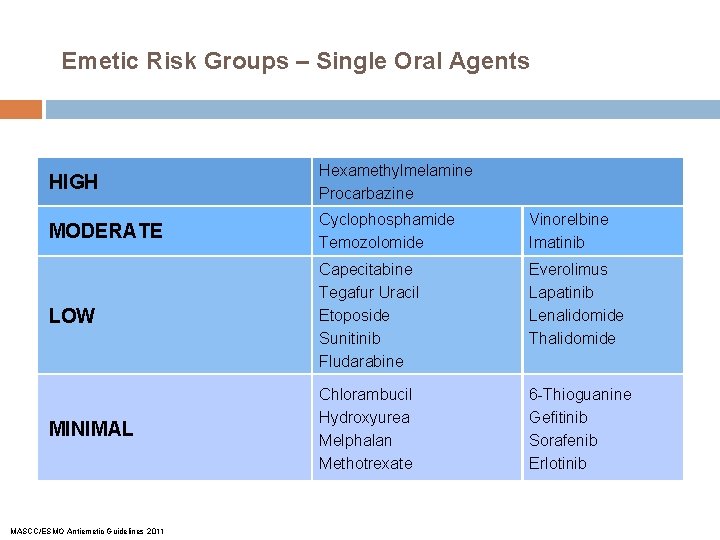
Emetic Risk Groups – Single Oral Agents HIGH Hexamethylmelamine Procarbazine MODERATE Cyclophosphamide Temozolomide Vinorelbine Imatinib LOW Capecitabine Tegafur Uracil Etoposide Sunitinib Fludarabine Everolimus Lapatinib Lenalidomide Thalidomide MINIMAL Chlorambucil Hydroxyurea Melphalan Methotrexate 6 -Thioguanine Gefitinib Sorafenib Erlotinib MASCC/ESMO Antiemetic Guidelines 2011

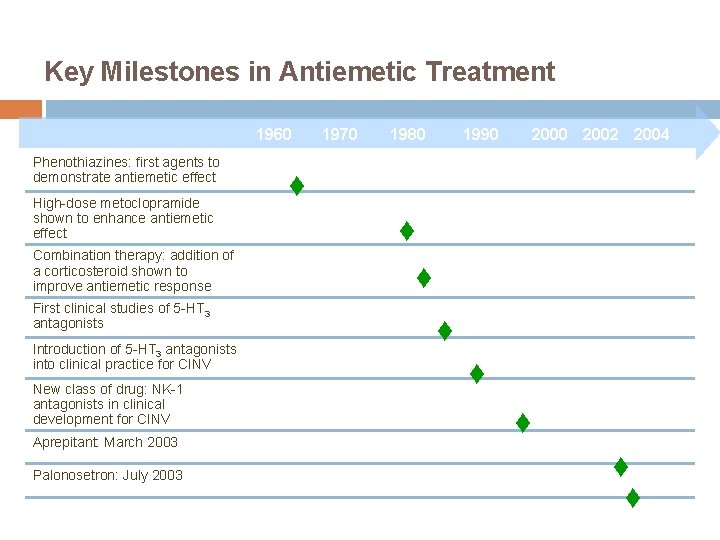
Key Milestones in Antiemetic Treatment 1960 Phenothiazines: first agents to demonstrate antiemetic effect High-dose metoclopramide shown to enhance antiemetic effect Combination therapy: addition of a corticosteroid shown to improve antiemetic response First clinical studies of 5 -HT 3 antagonists Introduction of 5 -HT 3 antagonists into clinical practice for CINV New class of drug: NK-1 antagonists in clinical development for CINV Aprepitant: March 2003 Palonosetron: July 2003 1970 1980 1990 2002 2004

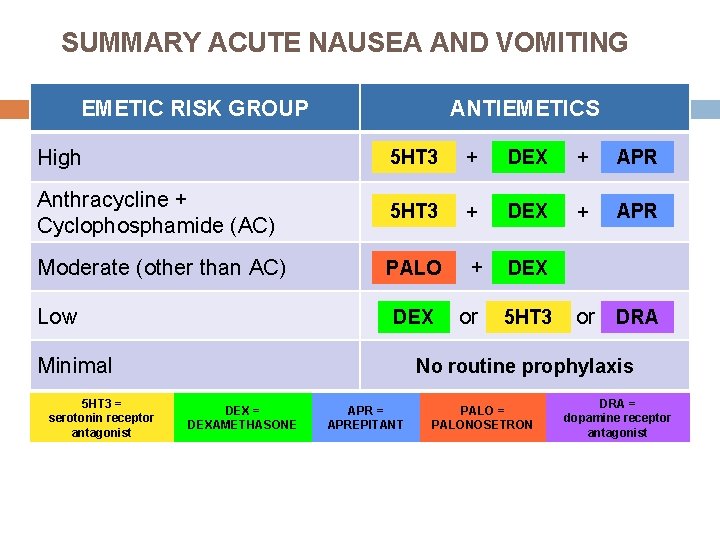
SUMMARY ACUTE NAUSEA AND VOMITING EMETIC RISK GROUP High ANTIEMETICS 5 HT 3 + Anthracycline + Cyclophosphamide (AC) + DEX APR + DEX + Moderate (other than AC) 5 HT 3 + PALO Low PALO + DEX or 5 HT 3 DEX + + + APR DEX or DRA DEX No routine prophylaxis Minimal 5 HT 3 = serotonin receptor antagonist DEX = DEXAMETHASONE APR = APREPITANT PALO = PALONOSETRON DRA = dopamine receptor antagonist * NOTE: If the NK 1 receptor antagonist is not available for AC chemotherapy, palonosetron is the preferred 5 -HT 3 receptor antagonist. The Antiemetic Subcommittee of The Multinational Association of Supportive Care in Cancer. - Ann Oncol 2010; www. mascc. org.

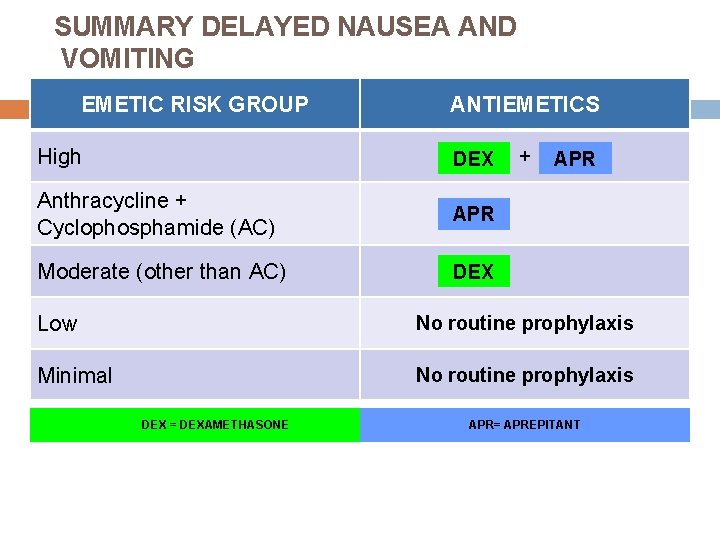
SUMMARY DELAYED NAUSEA AND VOMITING EMETIC RISK GROUP High ANTIEMETICS 5 HT 3 Anthracycline + Cyclophosphamide (AC) Moderate (other than AC) Low 5 HT 3 + DEX + APR DEX PALO + DEX No routine prophylaxis Minimal DEX = DEXAMETHASONE APR= APREPITANT The Antiemetic Subcommittee of The Multinational Association of Supportive Care in Cancer. Ann Oncol 2010; www. mascc. org.

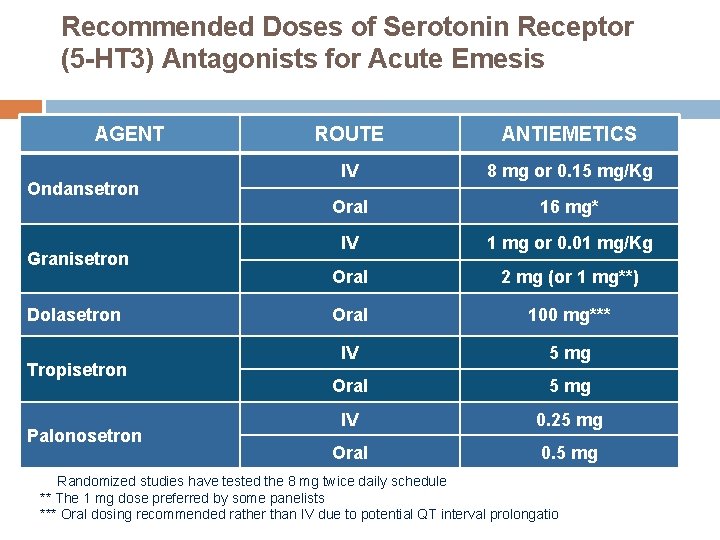
Recommended Doses of Serotonin Receptor (5 -HT 3) Antagonists for Acute Emesis AGENT Ondansetron Granisetron Dolasetron Tropisetron Palonosetron ROUTE IV 5 HT 3 ANTIEMETICS + 8 mg+ or 0. 15 mg/Kg DEX APR Oral IV 5 HT 3 Oral PALO Oral IV 16 mg* APR 1 mg+ or 0. 01 mg/Kg + DEX 2 mg (or 1 mg**) + DEX 100 mg*** 5 mg Oral 5 mg IV 0. 25 mg Oral 0. 5 mg * Randomized studies have tested the 8 mg twice daily schedule ** The 1 mg dose preferred by some panelists *** Oral dosing recommended rather than IV due to potential QT interval prolongation

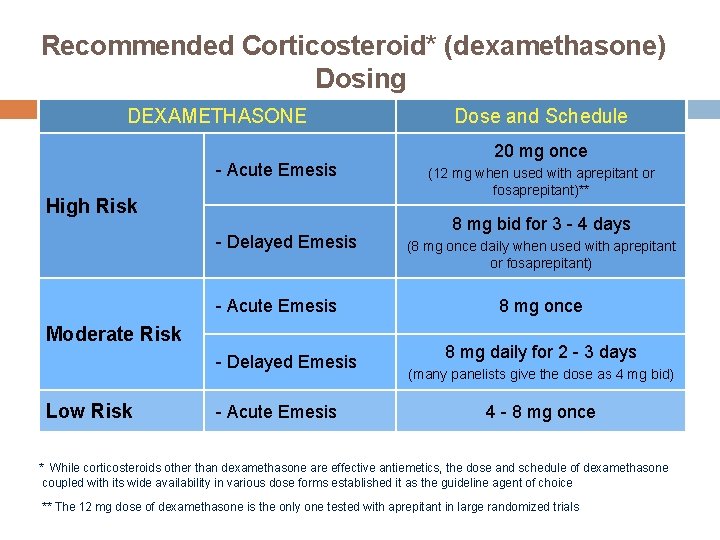
Recommended Corticosteroid* (dexamethasone) Dosing DEXAMETHASONE - Acute Emesis High Risk - Delayed Emesis - Acute Emesis Moderate Risk - Delayed Emesis Low Risk - Acute Emesis Dose and Schedule 20 mg once (12 mg when used with aprepitant or fosaprepitant)** 8 mg bid for 3 - 4 days (8 mg once daily when used with aprepitant or fosaprepitant) 8 mg once 8 mg daily for 2 - 3 days (many panelists give the dose as 4 mg bid) 4 - 8 mg once * While corticosteroids other than dexamethasone are effective antiemetics, the dose and schedule of dexamethasone coupled with its wide availability in various dose forms established it as the guideline agent of choice ** The 12 mg dose of dexamethasone is the only one tested with aprepitant in large randomized trials

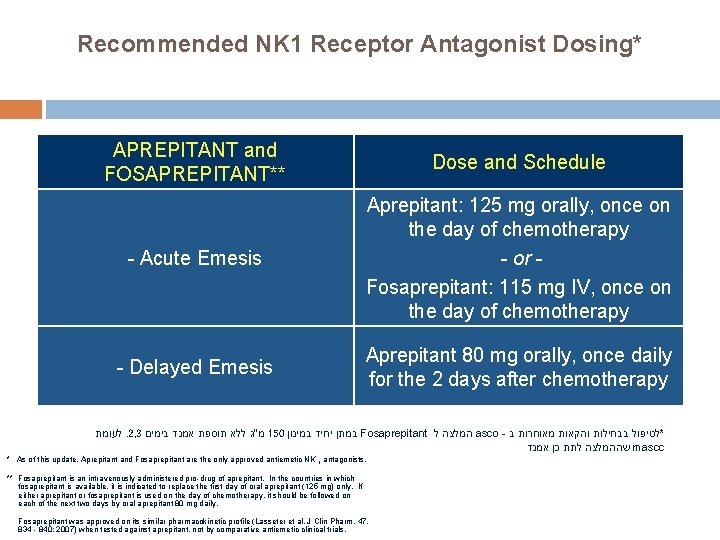
Recommended NK 1 Receptor Antagonist Dosing* APREPITANT and FOSAPREPITANT** Dose and Schedule - Acute Emesis Aprepitant: 125 mg orally, once on the day of chemotherapy - or Fosaprepitant: 115 mg IV, once on the day of chemotherapy - Delayed Emesis Aprepitant 80 mg orally, once daily for the 2 days after chemotherapy לעומת. 2, 3 מ"ג ללא תוספת אמנד בימים 150 במתן יחיד במינון Fosaprepitant המלצה ל asco - *לטיפול בבחילות והקאות מאוחרות ב שההמלצה לתת כן אמנד mascc * As of this update, Aprepitant and Fosaprepitant are the only approved antiemetic NK 1 antagonists. ** Fosaprepitant is an intravenously administered pro-drug of aprepitant. In the countries in which fosaprepitant is available, it is indicated to replace the first day of oral aprepitant (125 mg) only. If either aprepitant or fosaprepitant is used on the day of chemotherapy, it should be followed on each of the next two days by oral aprepitant 80 mg daily. Fosaprepitant was approved on its similar pharmacokinetic profile (Lasseter et al. J Clin Pharm. 47, 834 - 840; 2007) when tested against aprepitant, not by comparative antiemetic clinical trials.


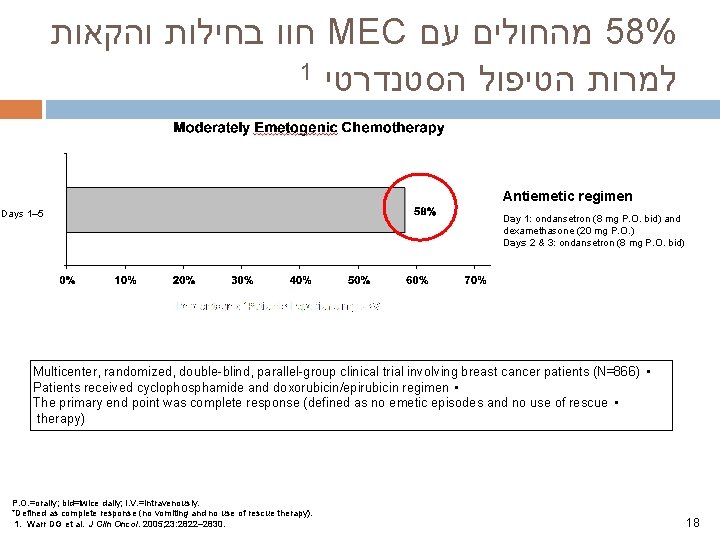
חוו בחילות והקאות MEC מהחולים עם 58% 1 למרות הטיפול הסטנדרטי Antiemetic regimen Days 1– 5 Day 1: ondansetron (8 mg P. O. bid) and dexamethasone (20 mg P. O. ) Days 2 & 3: ondansetron (8 mg P. O. bid) Multicenter, randomized, double-blind, parallel-group clinical trial involving breast cancer patients (N=866) • Patients received cyclophosphamide and doxorubicin/epirubicin regimen • The primary end point was complete response (defined as no emetic episodes and no use of rescue • therapy) P. O. =orally; bid=twice daily; I. V. =intravenously. *Defined as complete response (no vomiting and no use of rescue therapy). 1. Warr DG et al. J Clin Oncol. 2005; 23: 2822– 2830. 18




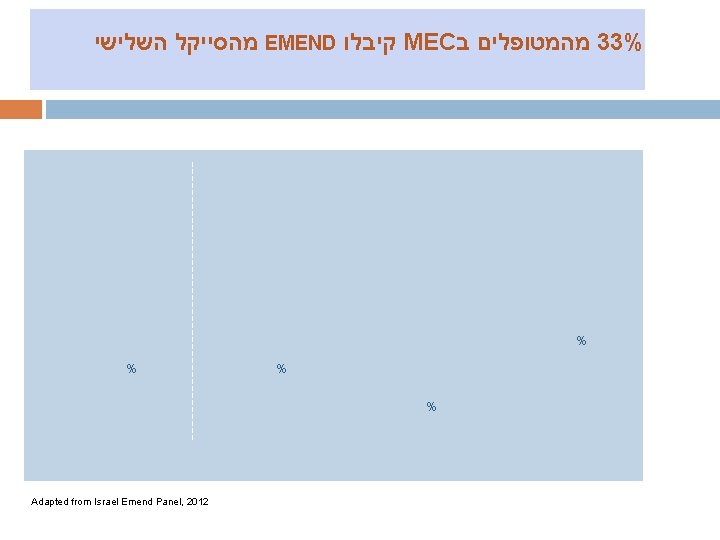
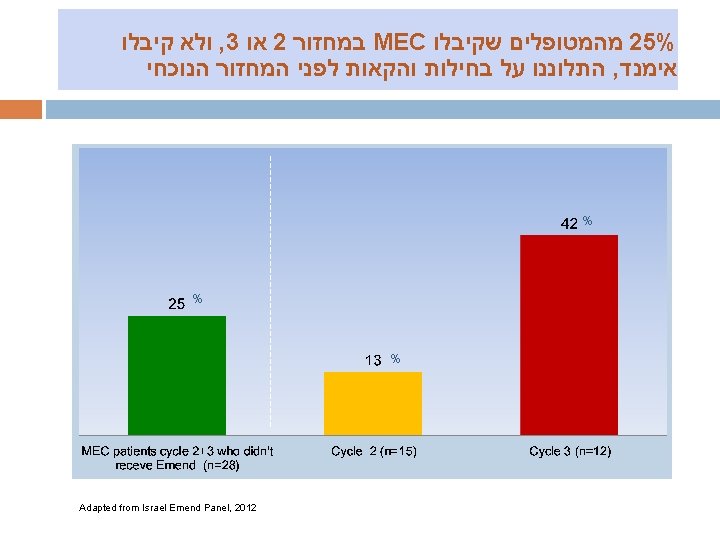
מהסייקל השלישי EMEND קיבלו MEC מהמטופלים ב 33% % % Adapted from Israel Emend Panel, 2012






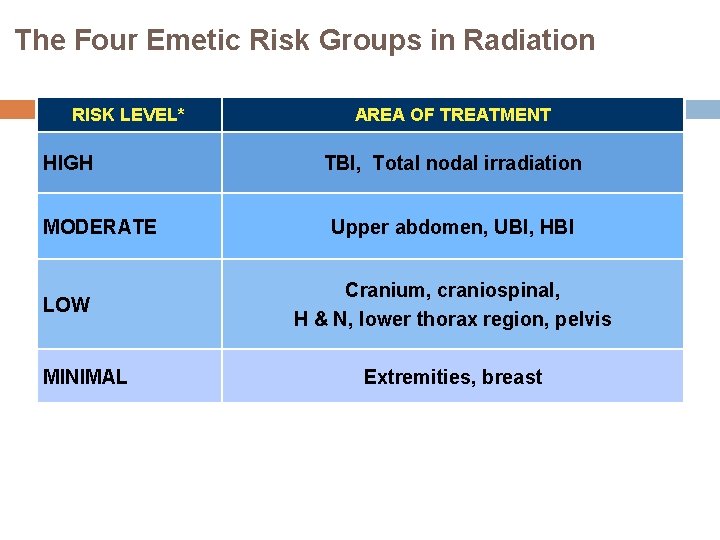
The Four Emetic Risk Groups in Radiation RISK LEVEL* HIGH MODERATE LOW MINIMAL AREA OF TREATMENT TBI, Total nodal irradiation Upper abdomen, UBI, HBI Cranium, craniospinal, H & N, lower thorax region, pelvis Extremities, breast TBI: total body irradiation, HBI: half body irradiation, UBI: upper body irradiation *in concomitant radiochemotherapy the antiemetic prophylaxis is according to the chemotherapy-related antiemetic guidelines of the corresponding risk category, unless the risk of emesis is higher with radiotherapy than chemotherapy

