The Foundations Classical Split and Splitless Injection Nicholas











- Slides: 30

The Foundations: Classical Split and Splitless Injection Nicholas H. Snow Department of Chemistry Seton Hall University South Orange, NJ 07079 snownich@shu. edu

Split and Splitless • Split – vaporize and remove most of the sample to waste • Splitless – vaporize and transfer most of the sample to the column; use cold trapping and solvent effects to focus bands • Both use the same hardware

Split Inlet • • • Use for higher concentration samples ppm and above hot inlet; vaporize sample mix with carrier gas use purge valve to “split” the sample – split ratio is critical • place fraction of sample on column

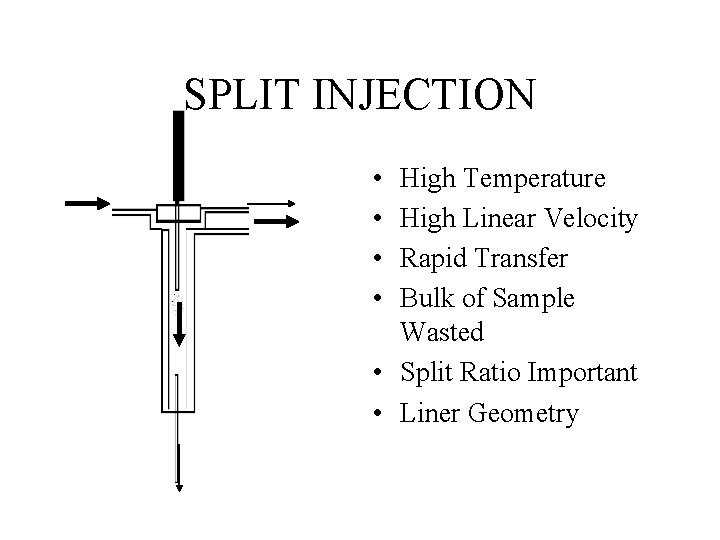
SPLIT INJECTION • • High Temperature High Linear Velocity Rapid Transfer Bulk of Sample Wasted • Split Ratio Important • Liner Geometry

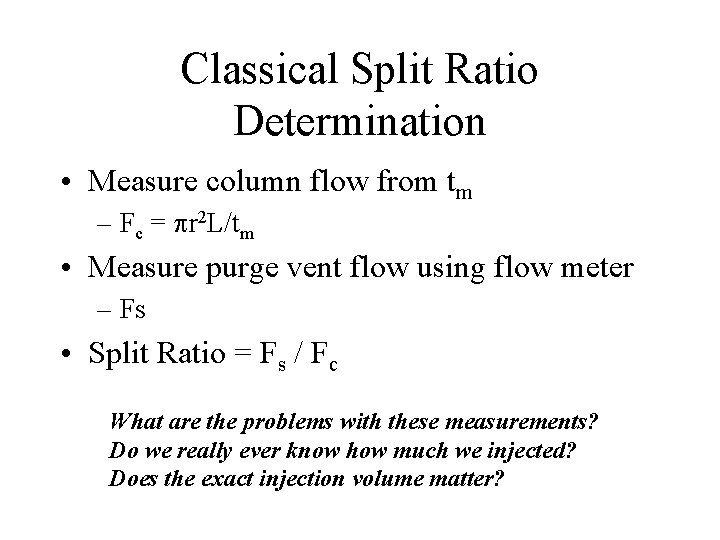
Classical Split Ratio Determination • Measure column flow from tm – Fc = pr 2 L/tm • Measure purge vent flow using flow meter – Fs • Split Ratio = Fs / Fc What are the problems with these measurements? Do we really ever know how much we injected? Does the exact injection volume matter?

Modern Split Ratio Determination • EPC systems measure pressures and flows directly • Column flow is calculated from inlet conditions and column dimensions – add equation here • Purge flow adjusted to desired value

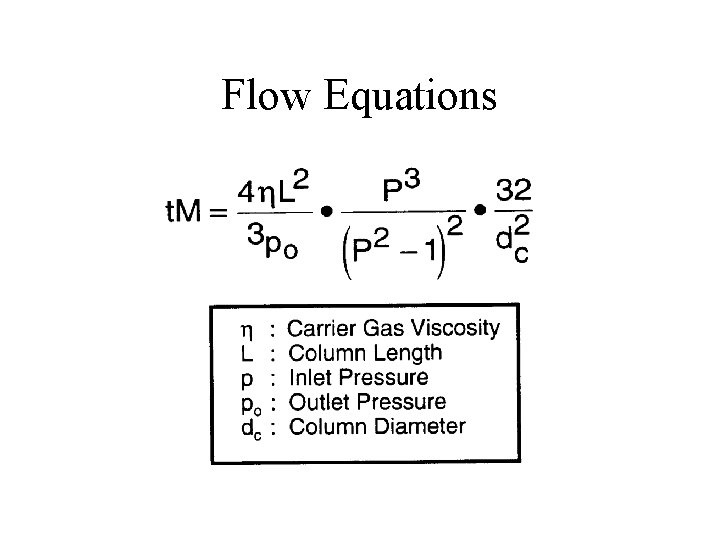
Flow Equations

Advantages of Split Inlets • • • Reduced sample size (narrow bands) Fast inlet flow rate (narrow bands) Dirty samples OK Simple to operate (best for isothermal GC) Inject “neat” samples Excellent interfacing

Disadvantages of Split Inlets • Nonlinear splitting – high molecular weights can be lost preferentially • Thermal degradation – hot metal surfaces can lead to reaction • Syringe needle discrimination • Trace analysis limited – ppm detection limits with FID

Split Injection Techniques • • Filled Needle Cold Needle Hot Needle Solvent Flush

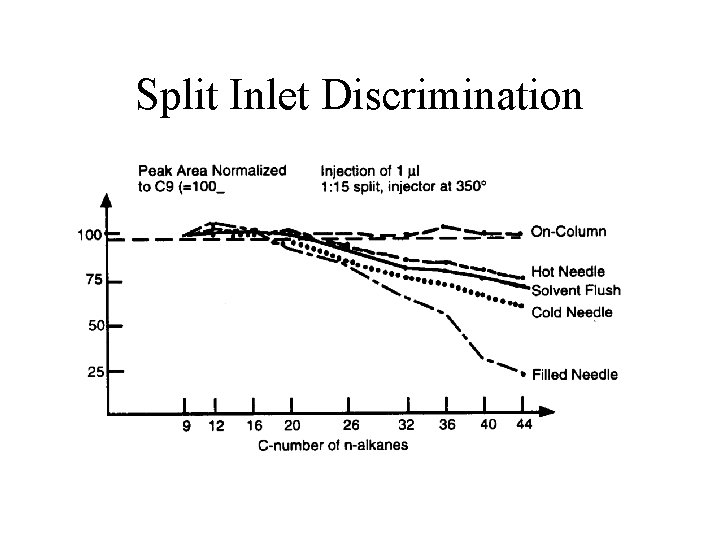
Split Inlet Discrimination

Summary - Split Inlet • Simple • Hot vaporizing technique – syringe discrimination (best to use autosampler) – liner discrimination • use glass wool (deactivated) • shape of liner may be critical • Best for “neat” or concentrated samples – high ppm or higher

Splitless Inlet • • Inject sample into hot inlet without “purge” 95% of sample enters column Same hardware as split except liner More variables – solvent, splitless time, initial column temperature • Open purge valve after short time • Better sensitivity

SPLITLESS INJECTION • • High Temperature Low Liner Velocity Slow Transfer Bulk of Sample and Solvent to Column • Many Factors Important

Steps in a Splitless Injection • Purge valve is off; column is cold • Inject sample – fast autosampler injection best – slower injections have been proposed • Flow through inlet is slow; slow transfer to cold column • After 30 -60 sec, open purge valve - cleans inlet • Temperature program column

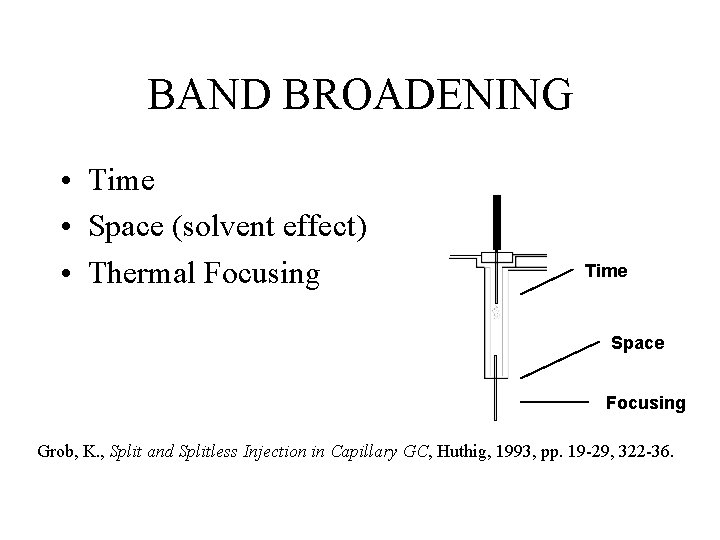
BAND BROADENING • Time • Space (solvent effect) • Thermal Focusing Time Space Focusing Grob, K. , Split and Splitless Injection in Capillary GC, Huthig, 1993, pp. 19 -29, 322 -36.

Band Focusing Mechanisms • Splitless injections involve slow transfer to column ---> initial peaks are broad • Need focusing – cold trap – solvent effects

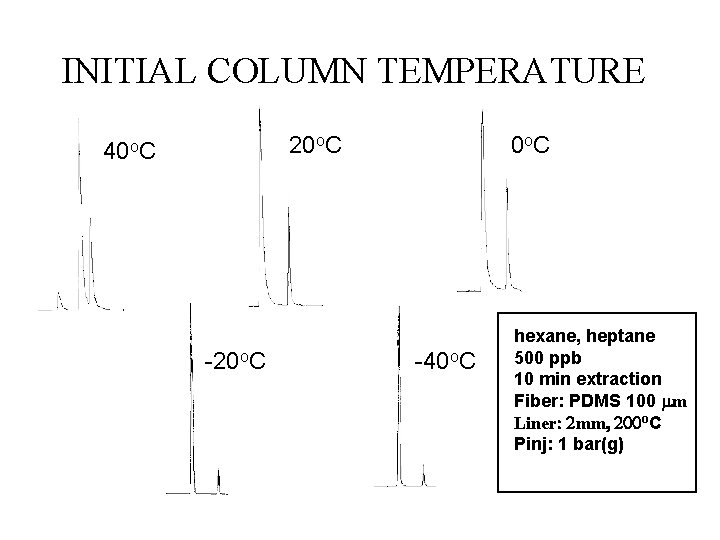
Cold Trap • Initial column temperature cold enough to “freeze” analyte on column

INITIAL COLUMN TEMPERATURE 20 o. C 40 o. C -20 o. C 0 o. C -40 o. C hexane, heptane 500 ppb 10 min extraction Fiber: PDMS 100 m Liner mm o. C Pinj: 1 bar(g)

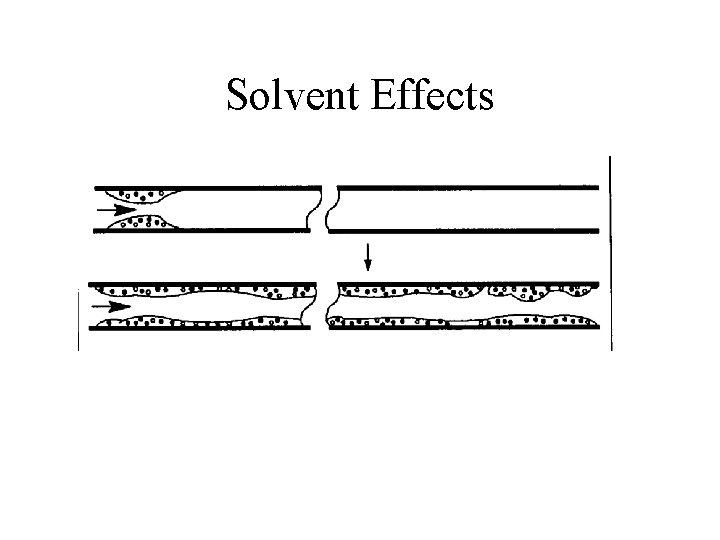
Solvent Effects • Solvent is recondensed in the column • Long plug of liquid • Start column 30 -50 degrees below normal boiling point of solvent

Solvent Effects

Solvent Effects • Refocus moderate volatility compounds near column head • Require solvent to wet stationary phase • Use non-polar solvent with non-polar stationary phase, etc.

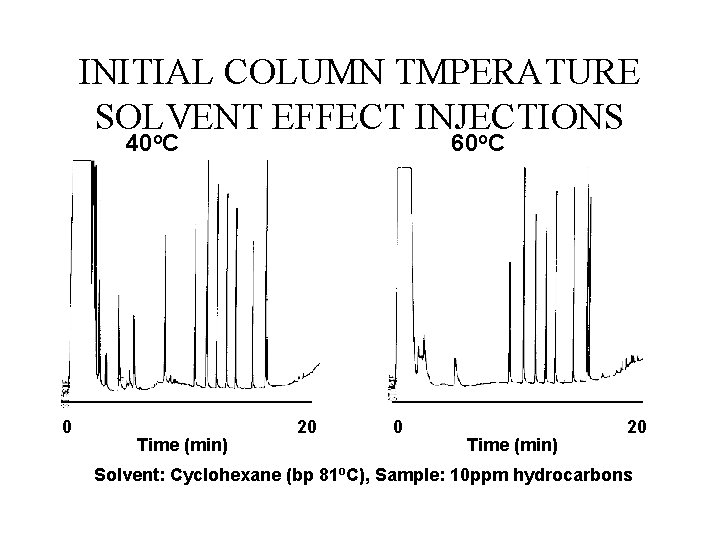
INITIAL COLUMN TMPERATURE SOLVENT EFFECT INJECTIONS o o 40 C 0 Time (min) 60 C 20 0 Time (min) 20 Solvent: Cyclohexane (bp 81 o. C), Sample: 10 ppm hydrocarbons

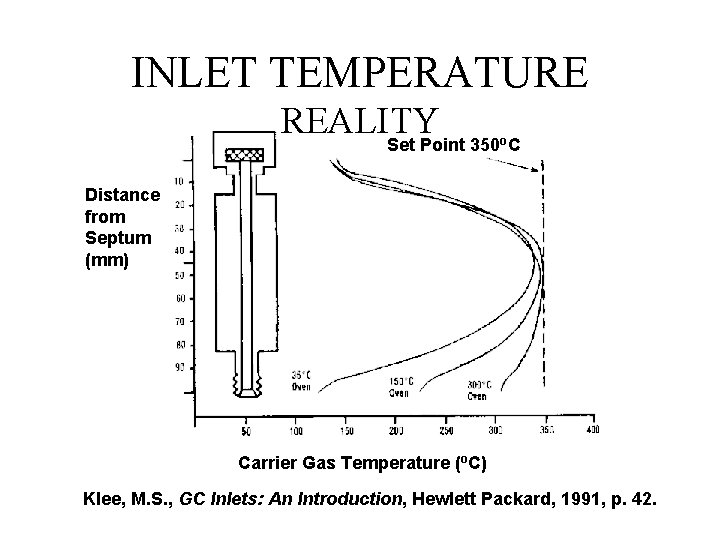
INLET TEMPERATURE REALITY Set Point 350 C o Distance from Septum (mm) Carrier Gas Temperature (o. C) Klee, M. S. , GC Inlets: An Introduction, Hewlett Packard, 1991, p. 42.

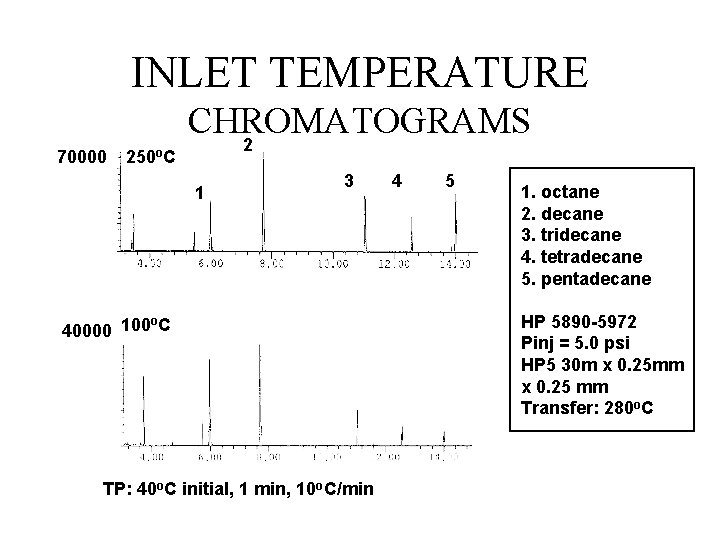
INLET TEMPERATURE 70000 250 o. C CHROMATOGRAMS 2 1 3 o 40000 100 C TP: 40 o. C initial, 1 min, 10 o. C/min 4 5 1. octane 2. decane 3. tridecane 4. tetradecane 5. pentadecane HP 5890 -5972 Pinj = 5. 0 psi HP 5 30 m x 0. 25 mm x 0. 25 mm Transfer: 280 o. C

INLET PRESSURE • Linear Gas Velocity Increased Injector Column • Analyte Boiling Point Increased

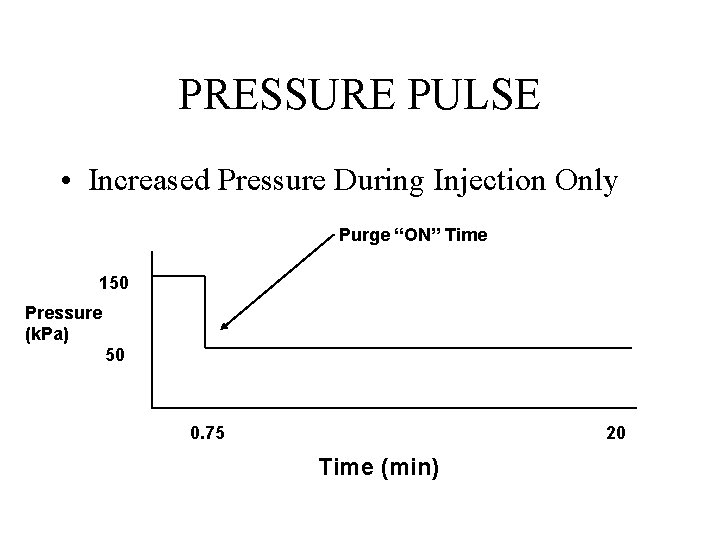
PRESSURE PULSE • Increased Pressure During Injection Only Purge “ON” Time 150 Pressure (k. Pa) 50 0. 75 20 Time (min)

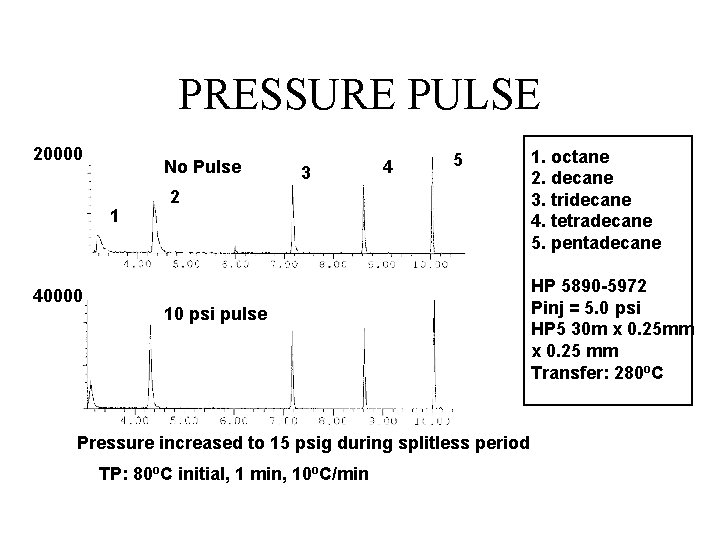
PRESSURE PULSE 20000 No Pulse 1 40000 3 4 5 2 10 psi pulse Pressure increased to 15 psig during splitless period TP: 80 o. C initial, 1 min, 10 o. C/min 1. octane 2. decane 3. tridecane 4. tetradecane 5. pentadecane HP 5890 -5972 Pinj = 5. 0 psi HP 5 30 m x 0. 25 mm x 0. 25 mm Transfer: 280 o. C

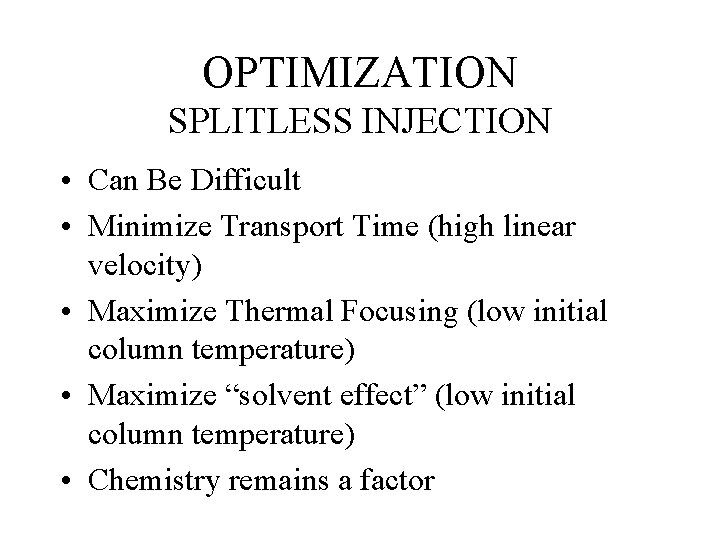
OPTIMIZATION SPLITLESS INJECTION • Can Be Difficult • Minimize Transport Time (high linear velocity) • Maximize Thermal Focusing (low initial column temperature) • Maximize “solvent effect” (low initial column temperature) • Chemistry remains a factor

REFERENCES • Grob, K. Split and Splitless Injection in Capillary GC, 3 rd. Edition, A. Huethig, 1993. • Klee, M. S. , GC Inlets: An Introduction, Hewlett Packard, 1991. • Stafford, S. S. , Electronic Pressure Control in Gas Chromatography, Hewlett Packard, 1993.