The Formation of Ionic Covalent Bonds Sections 1
The Formation of Ionic & Covalent Bonds Sections 1. 11 & 1. 12

Ionic Compounds l Have the following characteristics: l They are formed between a metal and a non- metal l They conduct electricity by forming an electrolyte (a compound that when dissolved in water produces a solution that conducts electricity) l They are have a strong crystal lattice structure l When mixed with water they dissociate, which means they break into their positive ion (cation) and negative ion (anion)
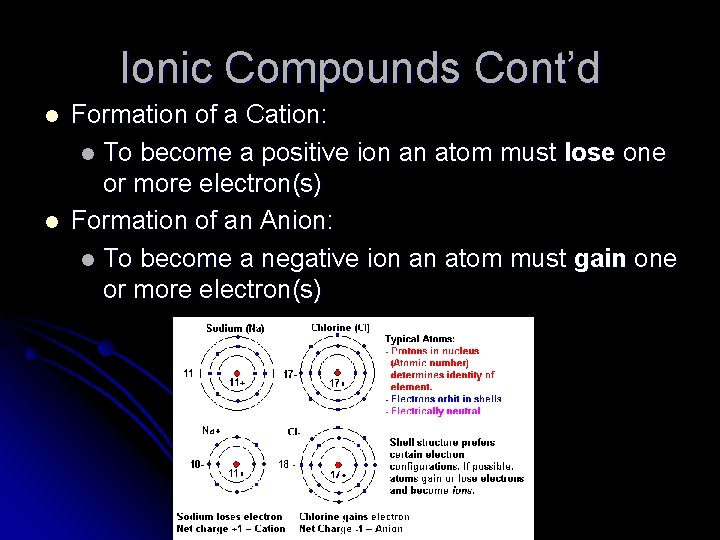
Ionic Compounds Cont’d l l Formation of a Cation: l To become a positive ion an atom must lose one or more electron(s) Formation of an Anion: l To become a negative ion an atom must gain one or more electron(s)

Naming Ionic Compounds

Covalent Compounds l Have the following characteristics: l Form between two non-metals l Involves the sharing of electrons l Form solutions that do not conduct electricity l Form brittle compounds
Lewis Structures l l Show the valence electrons for each atom (remember you can figure out the number of valence electrons from the periodic table) The valence electrons are represented as dots around the chemical symbol The maximum number of valence electrons is 8 (we call this an octet) You want to put electrons around the symbol so that they are unpaired first and then paired
Lewis Structures Cont’d l Examples: l Draw Lewis Structures for the following atoms Chlorine l Oxygen l Carbon l l Draw Lewis Structures for the following compounds (the goal is that each atom has a full octet) Two chlorine atoms l Two oxygen atoms l Carbon tetrahydride (methane) l
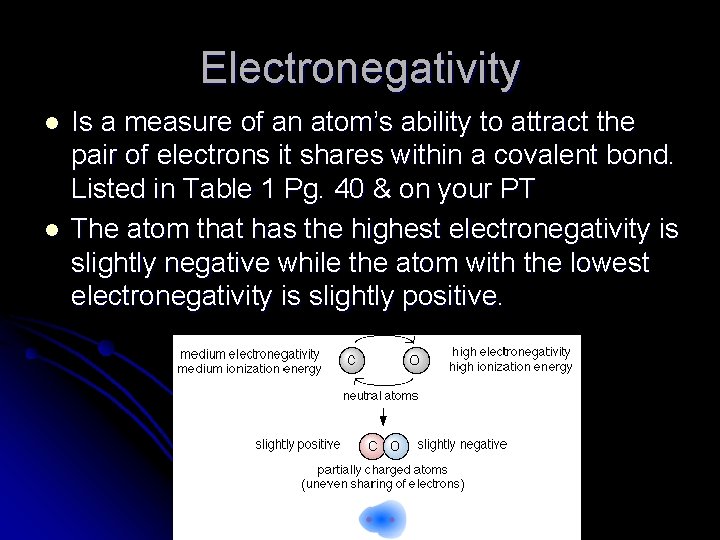
Electronegativity l l Is a measure of an atom’s ability to attract the pair of electrons it shares within a covalent bond. Listed in Table 1 Pg. 40 & on your PT The atom that has the highest electronegativity is slightly negative while the atom with the lowest electronegativity is slightly positive.
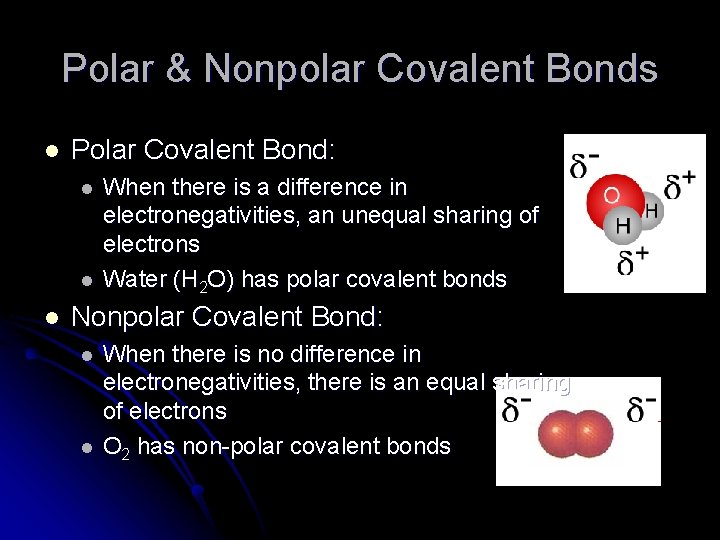
Polar & Nonpolar Covalent Bonds l Polar Covalent Bond: l l l When there is a difference in electronegativities, an unequal sharing of electrons Water (H 2 O) has polar covalent bonds Nonpolar Covalent Bond: l l When there is no difference in electronegativities, there is an equal sharing of electrons O 2 has non-polar covalent bonds
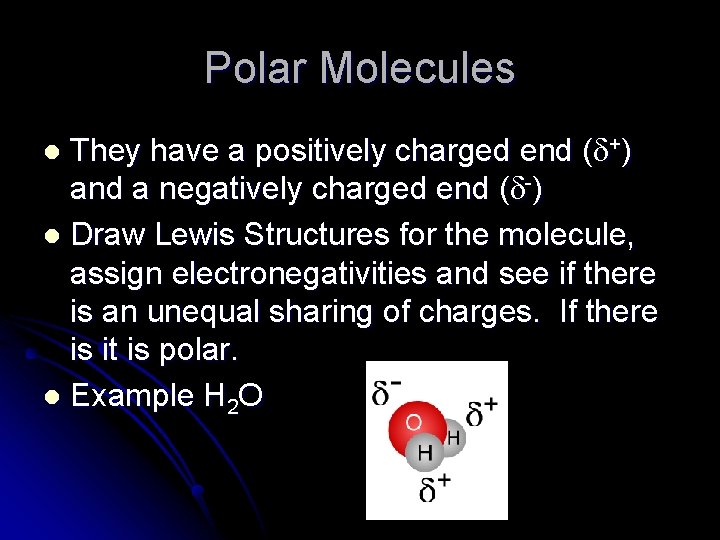
Polar Molecules They have a positively charged end ( +) and a negatively charged end ( -) l Draw Lewis Structures for the molecule, assign electronegativities and see if there is an unequal sharing of charges. If there is it is polar. l Example H 2 O l
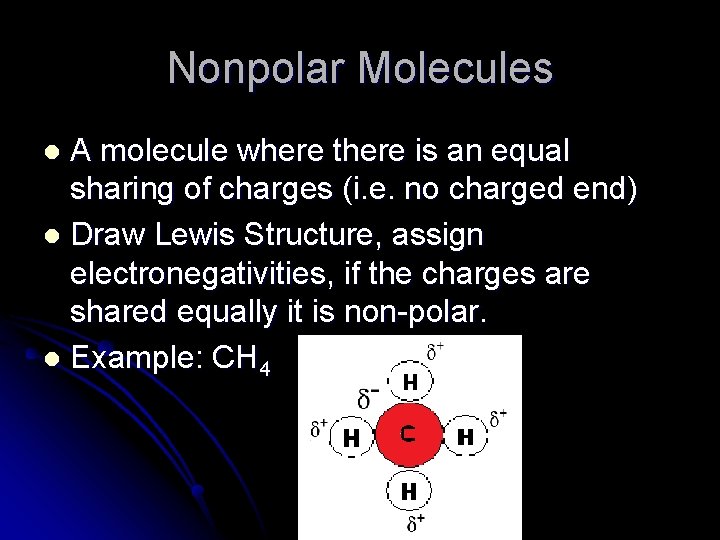
Nonpolar Molecules A molecule where there is an equal sharing of charges (i. e. no charged end) l Draw Lewis Structure, assign electronegativities, if the charges are shared equally it is non-polar. l Example: CH 4 l

Polar or Nonpolar? l NH 3 l CF 4 l CO l Cl 2
- Slides: 12