The FDA Approval Process for New Devices Roxana








- Slides: 19

The FDA Approval Process for New Devices Roxana Mehran, MD, MSCAI, FACC, FAHA, FESC Mount Sinai Professor Of Cardiovascular Clinical Research and Outcomes Professor of Medicine (Cardiology), and Population Health Science and Policy Director of Interventional Cardiovascular Research and Clinical Trials, Icahn School of Medicine at Mount Sinai, New York, NY, USA

Disclosures Affiliation/Financial Relationship Company Consultant/Advisory/Speaking Engagements Abbott Laboratories (to institution), Abiomed (spouse), Boston Scientific, Idorsia Pharmaceuticals Ltd. (no fee), Janssen, Medscape, Medtelligence (Janssen Scientific Affairs), Roivant Sciences Inc, Sanofi, Siemens Medical Solutions, Regeneron Pharmaceuticals (no fee), Spectranetics/Philips/Volcano Corp (to institution), The Medicines Company (spouse) Research Funding to Institution Abbott Laboratories, Abiomed, Applied Therapeutics, Astra. Zeneca, Bayer, Beth Israel Deaconess, BMS, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Medtronic, Novartis, Orbus. Neich Scientific Advisory Board Bristol-Myers Squibb (to institute), Medtelligence (Janssen Scientific Affairs), Merck (spouse) Equity, <1% Claret Medical, Elixir Medical DSMB membership paid to the institution Watermark Research Partners Associate Editor ACC, AMA

The FDA’s Mission “The FDA is responsible for protecting the public health by assuring the safety, efficacy and security of … medical devices” “The FDA is … responsible for advancing the public health by helping to speed innovations … and helping the public get accurate, science-based information”

Conflicting Criticisms of the FDA Loosen FDA Regulations! Drug and device approvals are too slow Tighten FDA Regulations! Too many drugs and devices are recalled each year Regulatory processes are under constant scrutiny to identify means of streamlining approval processes without compromising safety and efficacy, the primary mission of the agency.

Conflicting Criticisms of the FDA • The United States has arguably the most stringent regulations regarding approval of medical drugs and devices in the world • However, about 4, 500 FDA approved drugs and devices are pulled from U. S. shelves each year • In 2016 alone, manufacturers recalled 4, 448 drug and device products, according to the Center for Devices and Radiological Health and the Center for Drug Evaluation and Research • Of those recalls, the FDA classified 219 as Class I (i. e. potential to cause serious harm or death) Drugwatch, 2019

Criticisms of Drug & Device Approval § “Major drug recall list of the history suggest that lots of carelessness is involved during the drug development and manufacturing period. The long list of drug recall on FDA website is evidence that still industries are not following the standard guidelines issued by FDA. ” - Nagaich & Sadhna, Int J Pharm Investig 2015 § “Many high-risk medical devices today are approved on the basis of just one clinical trial (as opposed to new medications, which usually require two trials). And only a small minority of clinical studies of medical devices are randomized, controlled and blinded — the gold standard for reliable evidence (and the benchmark to which studies of drugs are held). ” - Redberg & Dhruva, NYT 2015

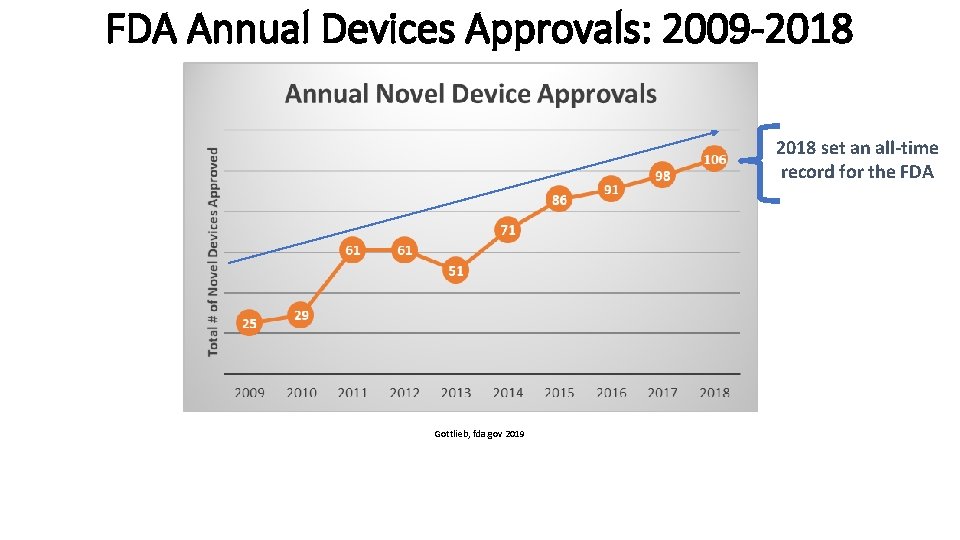
FDA Annual Devices Approvals: 2009 -2018 set an all-time record for the FDA Gottlieb, fda. gov 2019

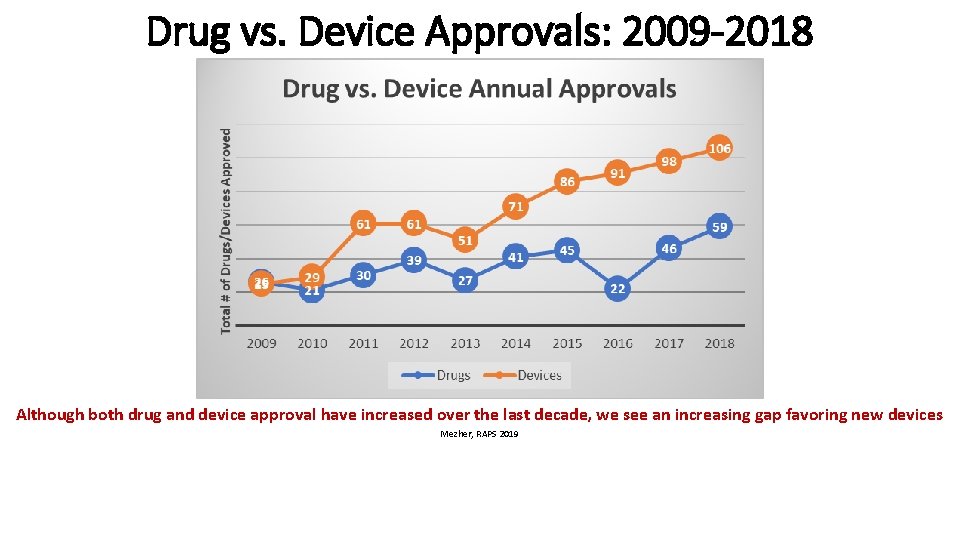
Drug vs. Device Approvals: 2009 -2018 Although both drug and device approval have increased over the last decade, we see an increasing gap favoring new devices Mezher, RAPS 2019

Steps to Get a New Product to Market

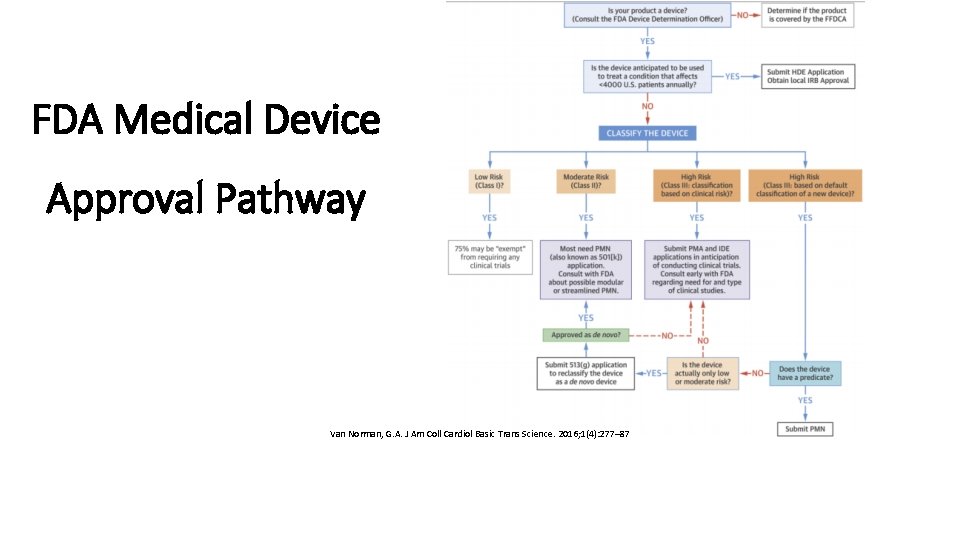
FDA Medical Device Approval Pathway Van Norman, G. A. J Am Coll Cardiol Basic Trans Science. 2016; 1(4): 277– 87

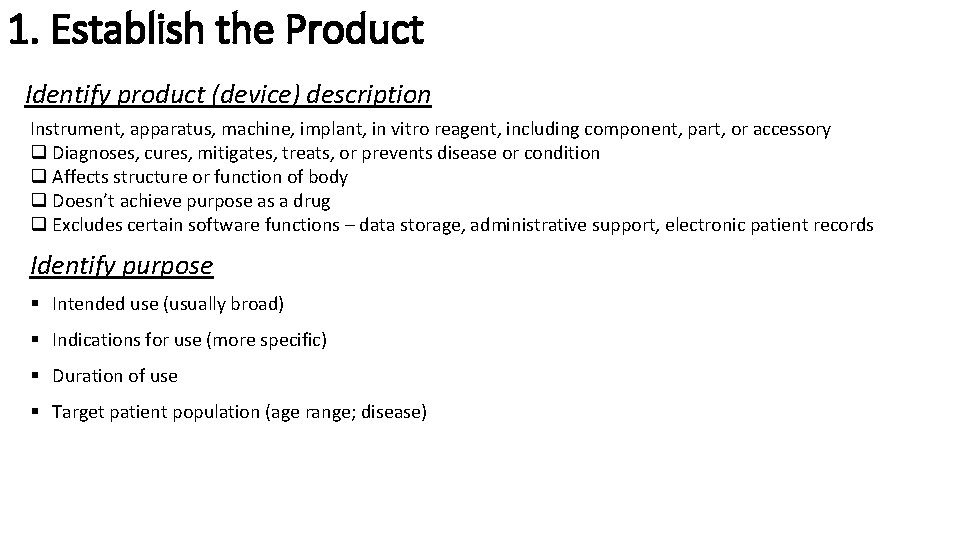
1. Establish the Product Identify product (device) description Instrument, apparatus, machine, implant, in vitro reagent, including component, part, or accessory q Diagnoses, cures, mitigates, treats, or prevents disease or condition q Affects structure or function of body q Doesn’t achieve purpose as a drug q Excludes certain software functions – data storage, administrative support, electronic patient records Identify purpose § Intended use (usually broad) § Indications for use (more specific) § Duration of use § Target patient population (age range; disease)

2. Verify that Product is Medical Device Treatment Diagnosis 3. Identify Classification and Regulatory Pathway § Identify regulatory classification § Classification will generally indicate regulatory pathway (premarket submission type) required for device

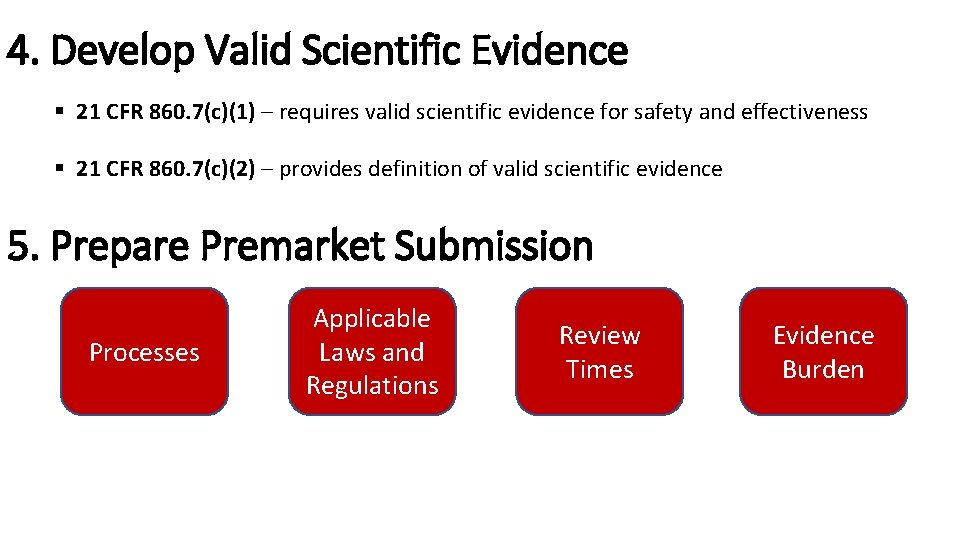
4. Develop Valid Scientific Evidence § 21 CFR 860. 7(c)(1) – requires valid scientific evidence for safety and effectiveness § 21 CFR 860. 7(c)(2) – provides definition of valid scientific evidence 5. Prepare Premarket Submission Processes Applicable Laws and Regulations Review Times Evidence Burden

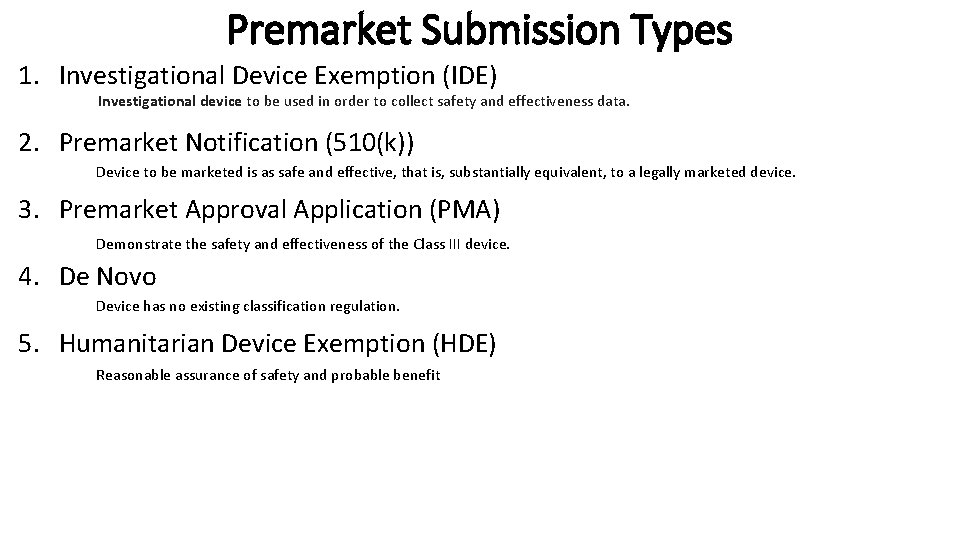
Premarket Submission Types 1. Investigational Device Exemption (IDE) Investigational device to be used in order to collect safety and effectiveness data. 2. Premarket Notification (510(k)) Device to be marketed is as safe and effective, that is, substantially equivalent, to a legally marketed device. 3. Premarket Approval Application (PMA) Demonstrate the safety and effectiveness of the Class III device. 4. De Novo Device has no existing classification regulation. 5. Humanitarian Device Exemption (HDE) Reasonable assurance of safety and probable benefit

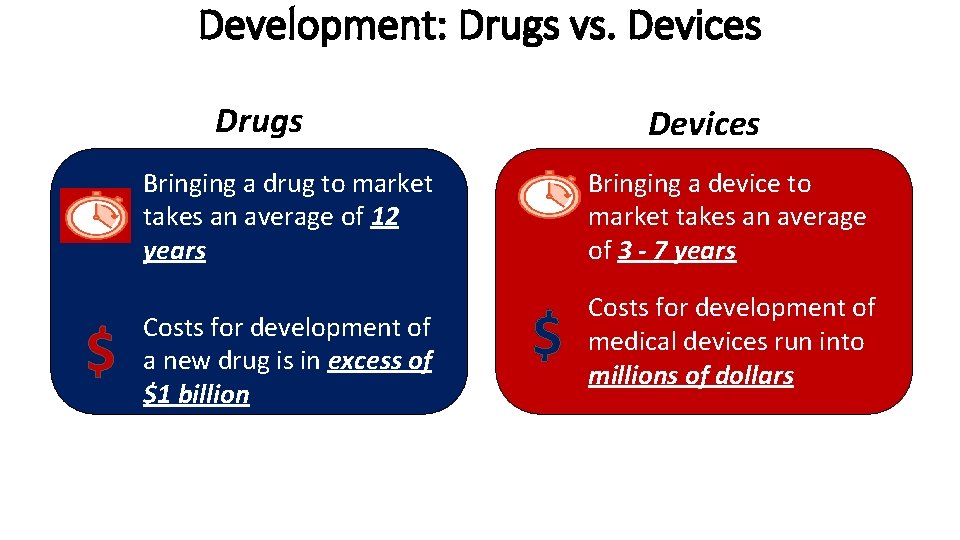
Development: Drugs vs. Devices Drugs Devices Bringing a drug to market takes an average of 12 years $ Costs for development of a new drug is in excess of $1 billion Bringing a device to market takes an average of 3 - 7 years $ Costs for development of medical devices run into millions of dollars

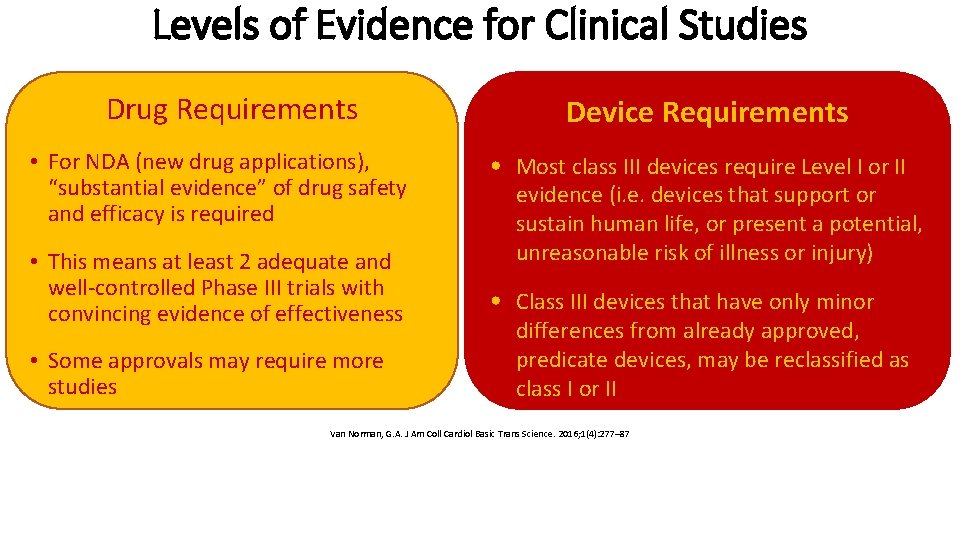
Levels of Evidence for Clinical Studies Drug Requirements • For NDA (new drug applications), “substantial evidence” of drug safety and efficacy is required • This means at least 2 adequate and well-controlled Phase III trials with convincing evidence of effectiveness • Some approvals may require more studies Device Requirements • Most class III devices require Level I or II evidence (i. e. devices that support or sustain human life, or present a potential, unreasonable risk of illness or injury) • Class III devices that have only minor differences from already approved, predicate devices, may be reclassified as class I or II Van Norman, G. A. J Am Coll Cardiol Basic Trans Science. 2016; 1(4): 277– 87

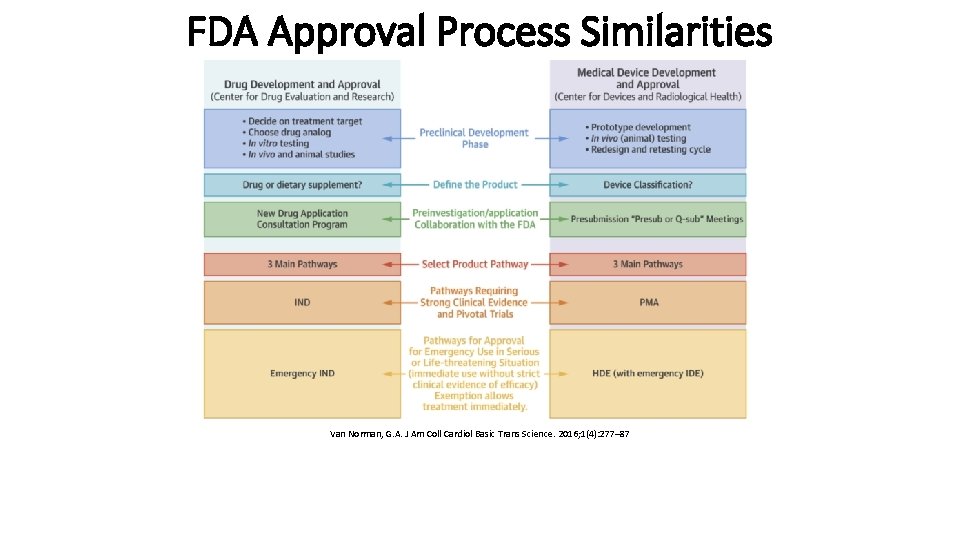
FDA Approval Process Similarities Van Norman, G. A. J Am Coll Cardiol Basic Trans Science. 2016; 1(4): 277– 87

Take Home Messages 1. Although the FDA has continued to make strides in streamlining new drug and device approval processes over the past several years, regulatory processes are often still criticized as being too tight as well as too loose. 2. The FDA must balance the rigor of its approval process (the most demanding in the world) with the needs of patients, without compromising safety or efficacy.

Thank You!