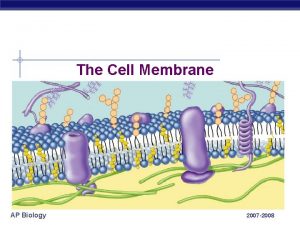
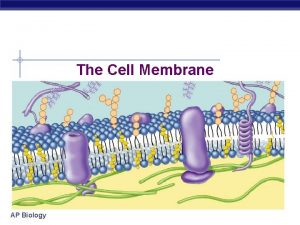
The Extracellular Matrix Jeff Miner 7717 Wohl Clinic



















- Slides: 69

The Extracellular Matrix Jeff Miner 7717 Wohl Clinic 362 -8235 minerj@wustl. edu

Suggested Reading **Overview: Lodish, Molecular Cell Biology (2008), Chapter 19, pp 801 -841. Lecture 1: The Extracellular Matrix **Review: A Aszodi, KR Legate, I Nakchbandi, and R. Fassler: What mouse mutants teach us about extracellular matrix function. Annu. Rev. Cell Dev. Biol. 22: 591 -621, 2006. **Review: JF Bateman, RP Boot-Handford, SR Lamandé: Genetic diseases of connective tissues: cellular and extracellular effets of ECM mutations. Nat. Rev. Genet. 10: 173 -183, 2009. KK Mc. Kee, D Harrison, S Capizzi, and PD Yurchenco: Role of laminin terminal globular domains in basement membrane assembly. J. Biol. Chem. 282: 21437 -21447, 2007. G Ge and DS Greenspan: BMP 1 controls TGFβ activation via cleavage of latent TGFβ-binding protein. J. Cell Biol. 175: 111 -120, 2006. Lecture 2: Cell-Matrix Interactions **Review: B-H Luo, CV Carman, and TA Springer: Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25: 619 -647, 2007. **Review: KR Legate, E Montanez, O Kudlacek, and Reinhard Fassler: ILK, PINCH, and parvin: the t. IPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 7: 20 -31, 2006. Review: Y Mao and JE Schwarzbauer: Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 24: 389 -399, 2005. **Review: R Barresi and KP Campbell: Dystroglycan: from biosynthesis to pathogenesis of human disease. J. Cell Sci. 119: 199 -207, 2006.

“Half of the secrets of the cell are outside the cell. ” Dr. Mina Bissell Oct. 17, 2007 Erlanger Auditorium

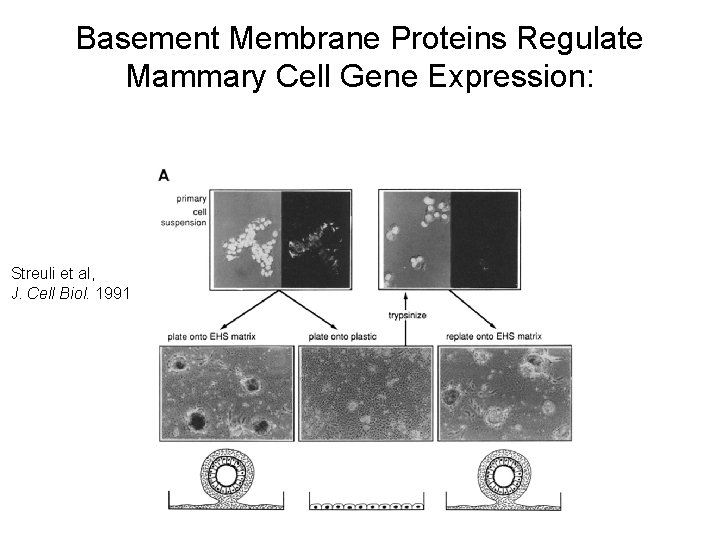
Basement Membrane Proteins Regulate Mammary Cell Gene Expression: Streuli et al, J. Cell Biol. 1991

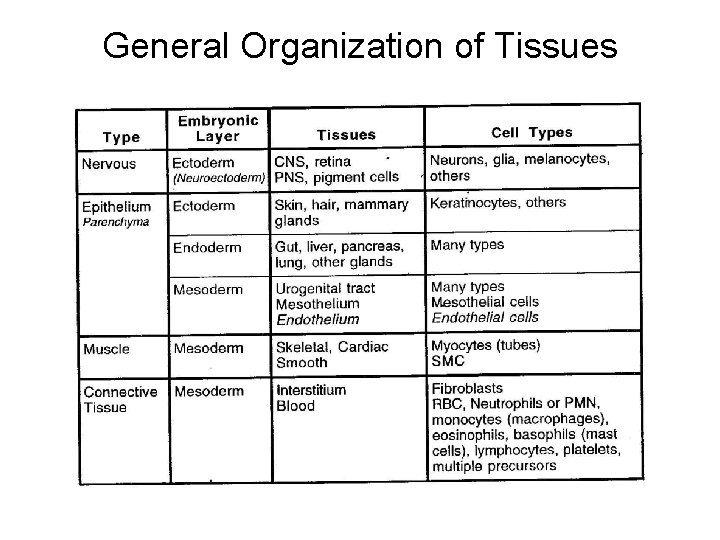
General Organization of Tissues There are

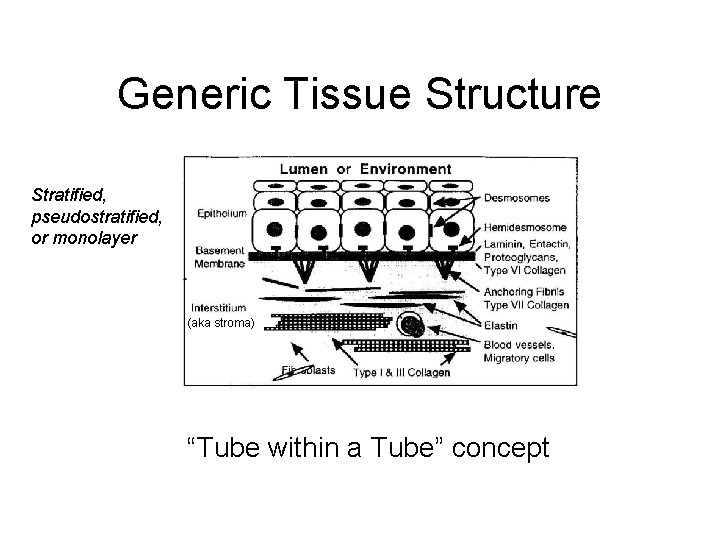
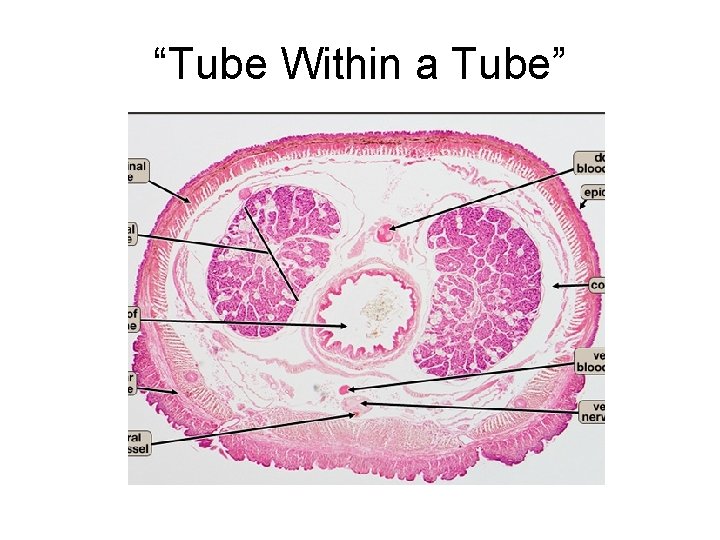
Generic Tissue Structure Stratified, pseudostratified, or monolayer (aka stroma) “Tube within a Tube” concept

“Tube Within a Tube”

Pancreas A Compartmentalized Tissue

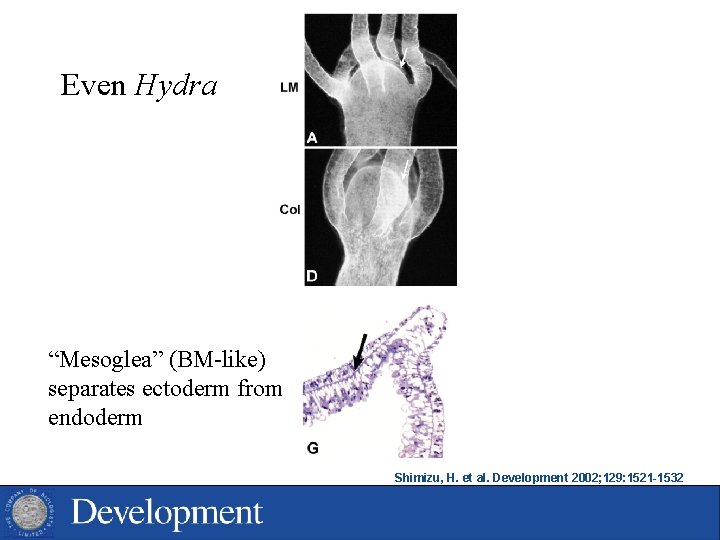
Even Hydra “Mesoglea” (BM-like) separates ectoderm from endoderm Shimizu, H. et al. Development 2002; 129: 1521 -1532

Why do all multicellular animals have ECM? • Act as structural support to maintain cell organization and integrity (epithelial tubes; mucosal lining of gut; skeletal muscle fiber integrity) • Compartmentalize tissues (pancreas: islets vs. exocrine component; skin: epidermis vs. dermis) • Provide hardness to bone and teeth (collagen fibrils become mineralized) • Present information to adjacent cells: – Inherent signals (e. g. , RGD motif in fibronectin) – Bound signals (BMP 7, TGFβ, FGF, SHH) • Serve as a highway for cell migration during development (neural crest migration), in normal tissue maintenance (intestinal mucosa), and in injury or disease (wound healing; cancer)

Types of ECMs • Basement membrane (basal lamina) – Epithelia, endothelia, muscle, fat, nerves • Elastic fibers – Skin, lung, large blood vessels • Stromal or interstitial matrix • Bone, tooth, and cartilage • Tendon and ligament

Cells Need Receptors to Recognize and Respond to ECM • • • Integrins Dystroglycan Syndecans Muscle-Specific kinase (Mu. SK) Others

Types of ECM Components • Collagens • Proteoglycans – Perlecan, aggrecan, agrin, collagen XVIII • Hyaluronan (no protein core) • Large Glycoproteins – Laminins, nidogens, fibronectin, vitronectin • Fibrillins, elastin, LTBPs, MAGPs, fibulins • “Matricellular” Proteins – SPARC, Thrombospondins, Osteopontin, tenascins

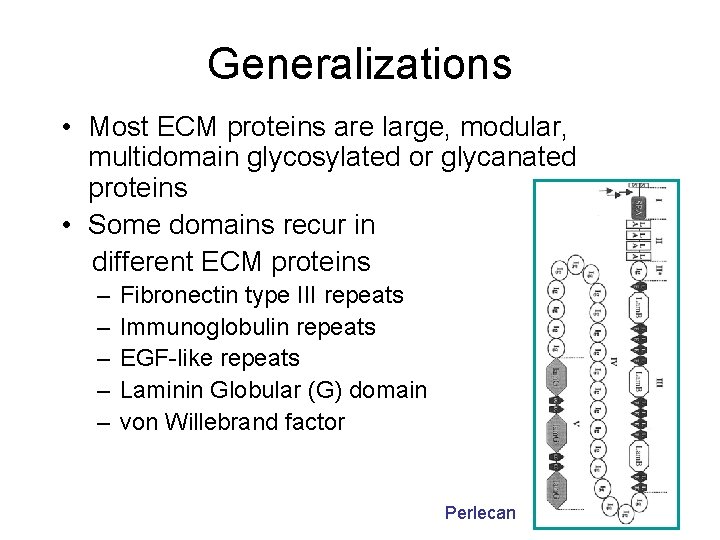
Generalizations • Most ECM proteins are large, modular, multidomain glycosylated or glycanated proteins • Some domains recur in different ECM proteins – – – Fibronectin type III repeats Immunoglobulin repeats EGF-like repeats Laminin Globular (G) domain von Willebrand factor Perlecan

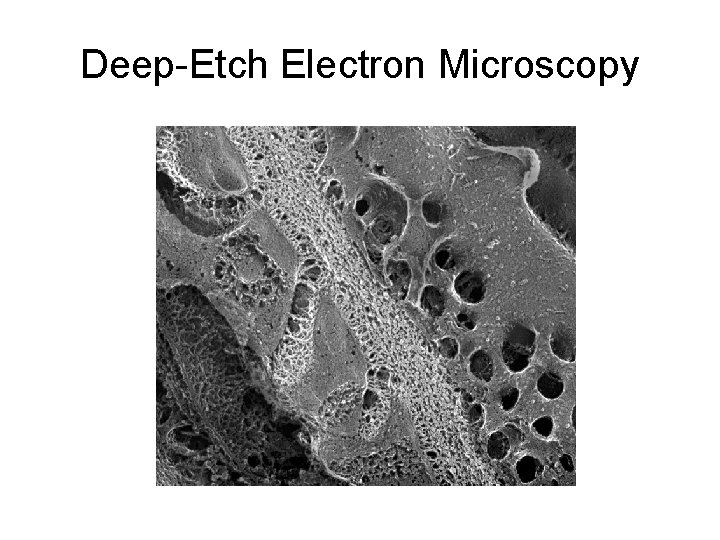
Basement Membranes • Specialized layers of extracellular matrix surrounding or adjacent to all epithelia, endothelia, peripheral nerves, muscle cells, and fat cells • Originally defined by electron microscopy as ribbon-like extracellular structures beneath epithelial cells

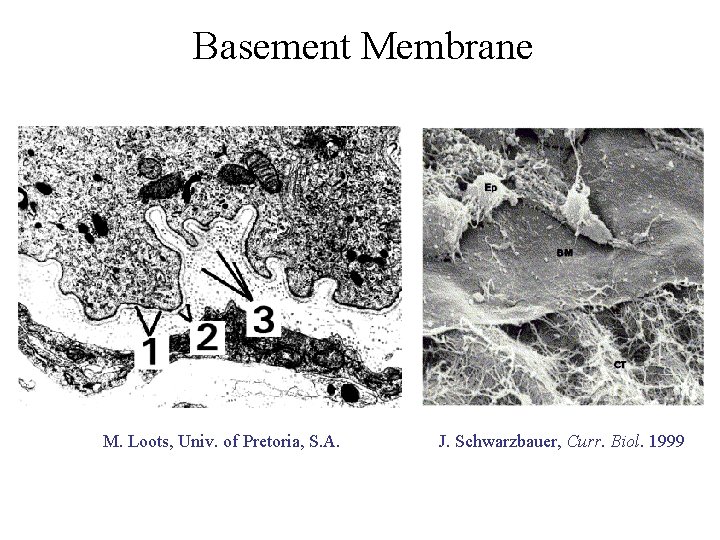
Basement Membrane M. Loots, Univ. of Pretoria, S. A. J. Schwarzbauer, Curr. Biol. 1999

Lamina Densa + Lamina Lucidae Kidney Glomerular Basement Membrane

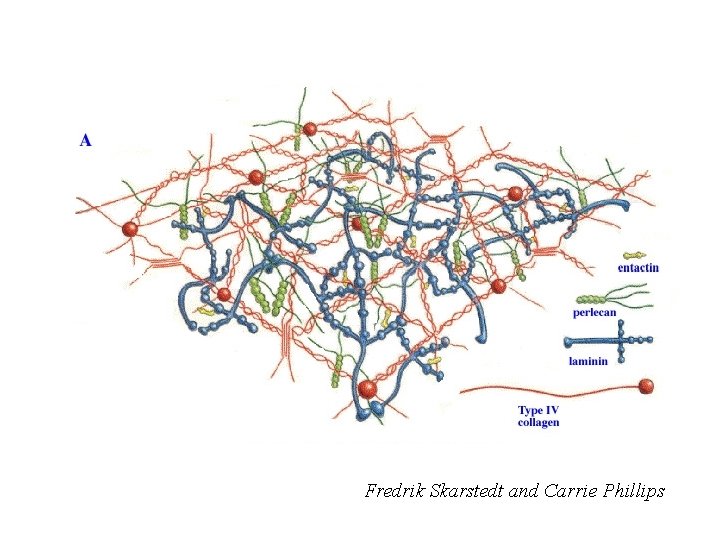
Fredrik Skarstedt and Carrie Phillips

Deep-Etch Electron Microscopy

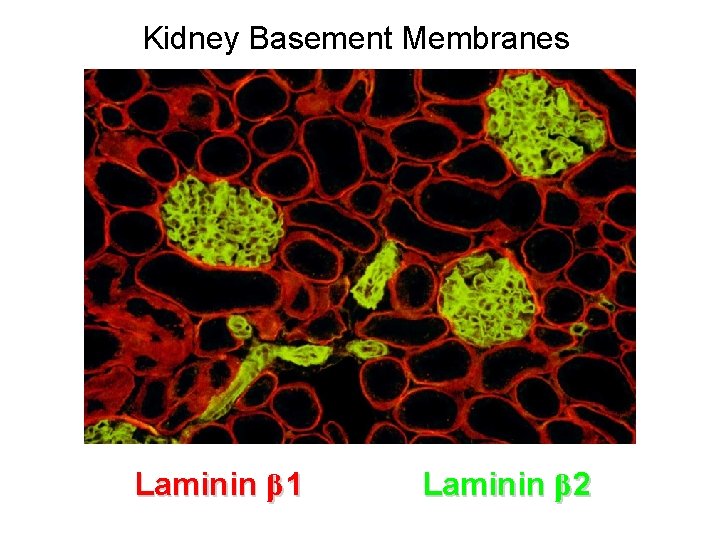
Basement Membranes • In general, basement membranes appear very similar to each other by EM. • But all are not alike! • There is a wealth of molecular and functional heterogeneity among basement membranes, due primarily to isoform variations of basement membrane components.

Kidney Basement Membranes Laminin β 1 Laminin β 2

Basement Membranes are Involved in a Multitude of Biological Processes • Influence cell proliferation, differentiation, and migration • Maintain cell polarization and organization, as well as tissue structure • Act as a filtration barrier in the kidney between the vasculature and the urinary space • Separate epithelia from the underlying stroma/mesenchyme/interstitium, which contains a non-basement membrane matrix

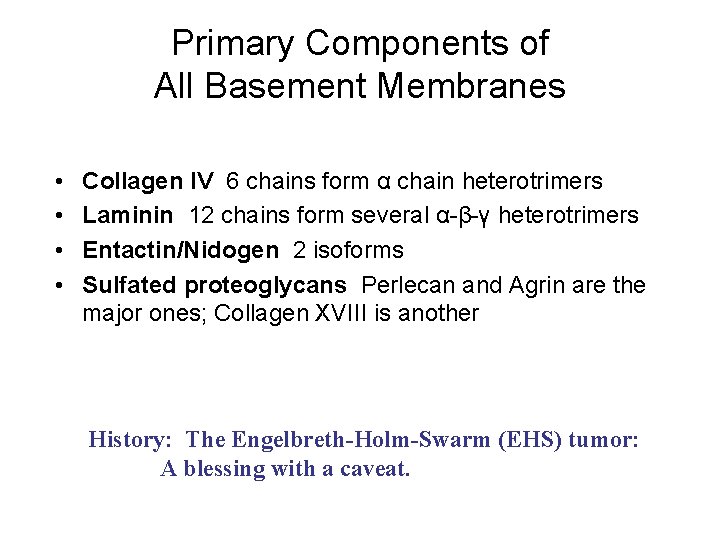
Primary Components of All Basement Membranes • • Collagen IV 6 chains form α chain heterotrimers Laminin 12 chains form several α-β-γ heterotrimers Entactin/Nidogen 2 isoforms Sulfated proteoglycans Perlecan and Agrin are the major ones; Collagen XVIII is another History: The Engelbreth-Holm-Swarm (EHS) tumor: A blessing with a caveat.

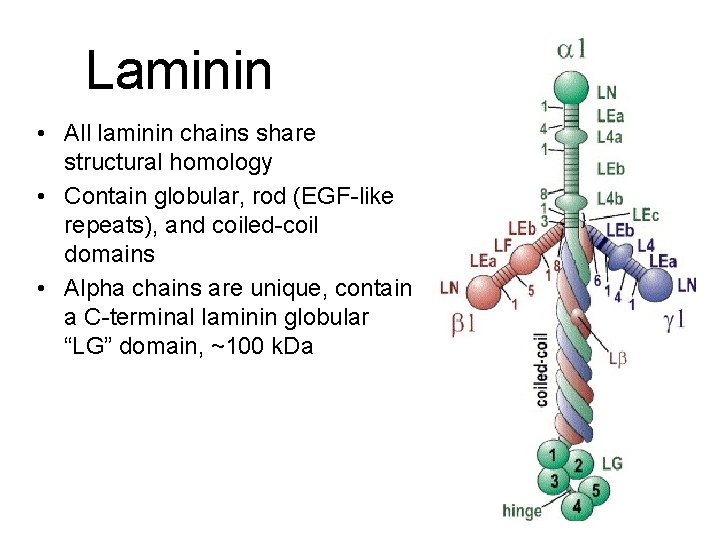
Laminin Heterotrimers are composed of one α, one β, and one γ chain. • 400 to 800 k. Da cruciform, Y, or rodshaped macromolecules. • Major glycoprotein of basement membranes—it’s required! • Chains are evolutionarily related. • 5 alpha, 4 beta, and 3 gamma chains are known. They assemble with each other non-randomly. • 15 heterotrimers described to date. LM-521

Laminin • All laminin chains share structural homology • Contain globular, rod (EGF-like repeats), and coiled-coil domains • Alpha chains are unique, contain a C-terminal laminin globular “LG” domain, ~100 k. Da (New nomenclature)

The Laminin Trimers Miner and Yurchenco, 2004

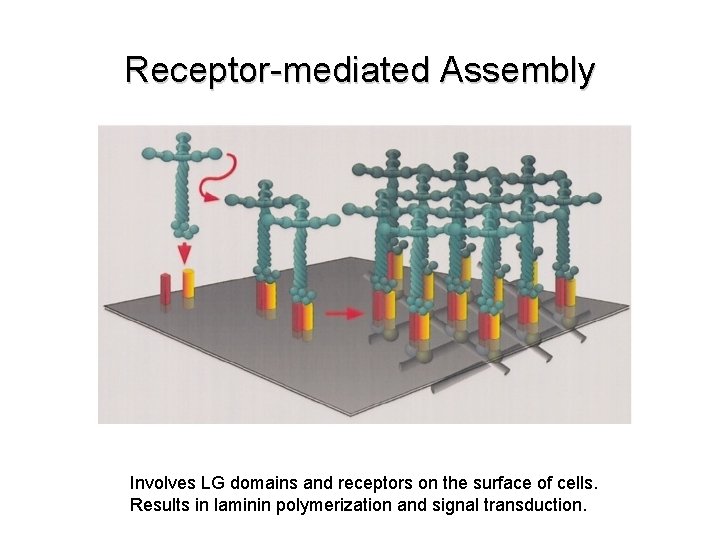
Laminin Trimers Polymerize • Laminin chains assemble into trimers in the ER and are secreted as trimers into the extracellular space. • Full-sized laminin trimers can self-polymerize into a macromolecular network through short arm-short arm interactions. • The α chain LG domain is left free for interactions with cellular receptors.

Receptor-mediated Assembly Involves LG domains and receptors on the surface of cells. Results in laminin polymerization and signal transduction.

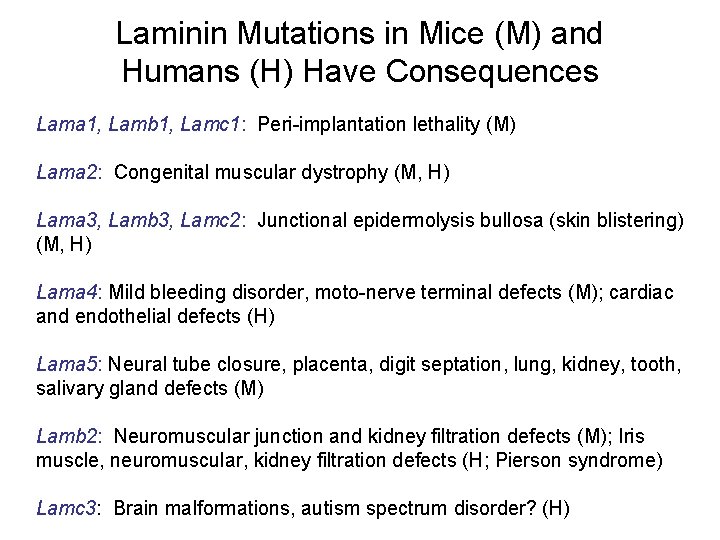
Laminin Mutations in Mice (M) and Humans (H) Have Consequences Lama 1, Lamb 1, Lamc 1: Peri-implantation lethality (M) Lama 2: Congenital muscular dystrophy (M, H) Lama 3, Lamb 3, Lamc 2: Junctional epidermolysis bullosa (skin blistering) (M, H) Lama 4: Mild bleeding disorder, moto-nerve terminal defects (M); cardiac and endothelial defects (H) Lama 5: Neural tube closure, placenta, digit septation, lung, kidney, tooth, salivary gland defects (M) Lamb 2: Neuromuscular junction and kidney filtration defects (M); Iris muscle, neuromuscular, kidney filtration defects (H; Pierson syndrome) Lamc 3: Brain malformations, autism spectrum disorder? (H)

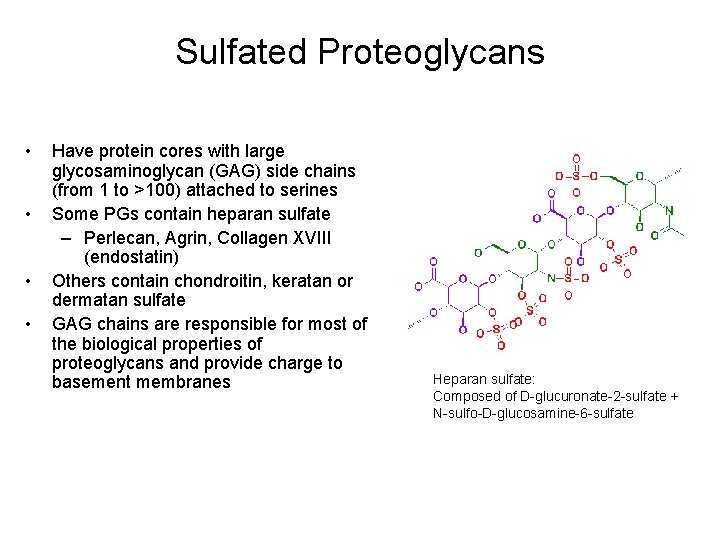
Sulfated Proteoglycans • • Have protein cores with large glycosaminoglycan (GAG) side chains (from 1 to >100) attached to serines Some PGs contain heparan sulfate – Perlecan, Agrin, Collagen XVIII (endostatin) Others contain chondroitin, keratan or dermatan sulfate GAG chains are responsible for most of the biological properties of proteoglycans and provide charge to basement membranes Heparan sulfate: Composed of D-glucuronate-2 -sulfate + N-sulfo-D-glucosamine-6 -sulfate

Some Major Proteoglycan Family Members From: Iozzo, R. V. (1998) Ann. Rev. Biochem. 67: 609 From: Iozzo, R. V. (2001) J. Clinic. Invest. 108: 165

Perlecan • Found widely in basement membranes and in cartilage. • Contains domains similar to LDL receptor, laminin, and N-CAM • Binds to Collagen IV and to Entactin/Nidogen

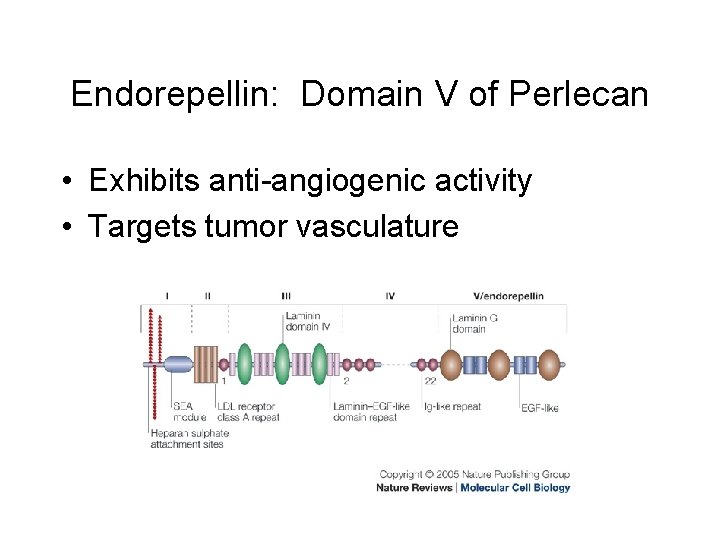
Endorepellin: Domain V of Perlecan • Exhibits anti-angiogenic activity • Targets tumor vasculature

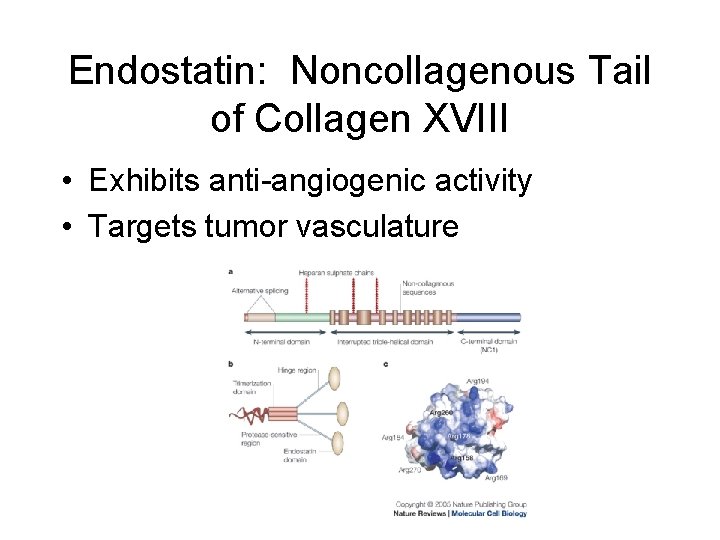
Endostatin: Noncollagenous Tail of Collagen XVIII • Exhibits anti-angiogenic activity • Targets tumor vasculature

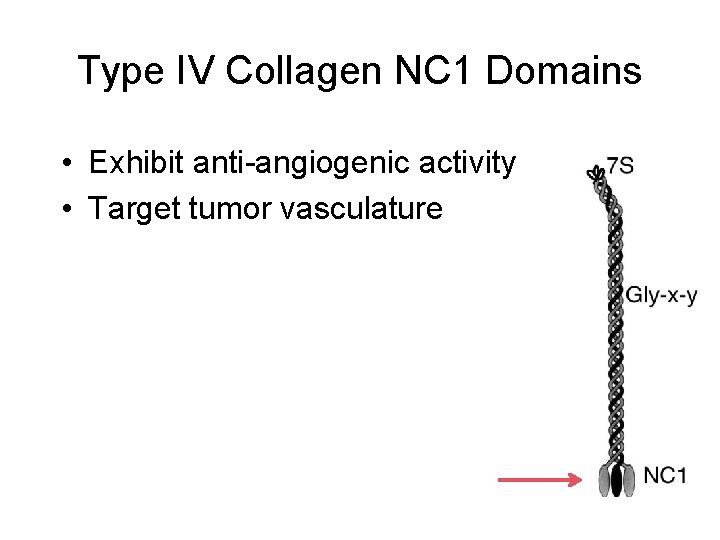
Type IV Collagen NC 1 Domains • Exhibit anti-angiogenic activity • Target tumor vasculature

Proteases Release Anti-Cancer Peptides Laminin cleavages MMP = Matrix Metalloproteinase MT-MMP = Membrane Tethered MMP From Zent and Pozzi, 2005

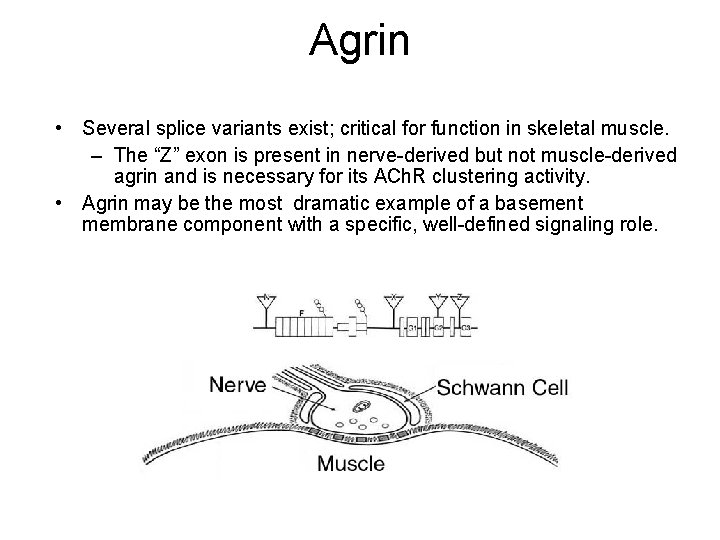
Agrin • A HSPG found widely in basement membranes • A modular protein containing domains homologous to follistatin, laminin, and perlecan • Isolated due to its ability to cluster pre-existing acetylcholine receptors in skeletal muscle fibers • BM form binds to laminin

Agrin • Several splice variants exist; critical for function in skeletal muscle. – The “Z” exon is present in nerve-derived but not muscle-derived agrin and is necessary for its ACh. R clustering activity. • Agrin may be the most dramatic example of a basement membrane component with a specific, well-defined signaling role.

The Collagens • The most ubiquitous structural protein. Characterized as a triple helical protein containing peptide chains with repeating Gly-Xaa-Yaa (usually Pro) triplets. • The triple helix forms through the association of three related polypeptides (α-chains) forming a coiled coil, with the side chain of every third residue directed towards the center of the superhelix. Steric constraints dictate that the center of the helix be occupied only by Glycine residues. • Many Proline and Lysine residues are enzymatically converted to hydroxyproline and hydroxylysine. • ~28 distinct collagen types; each is assigned a Roman numeral that generally delineates the chronological order in which the collagens were isolated/characterized.

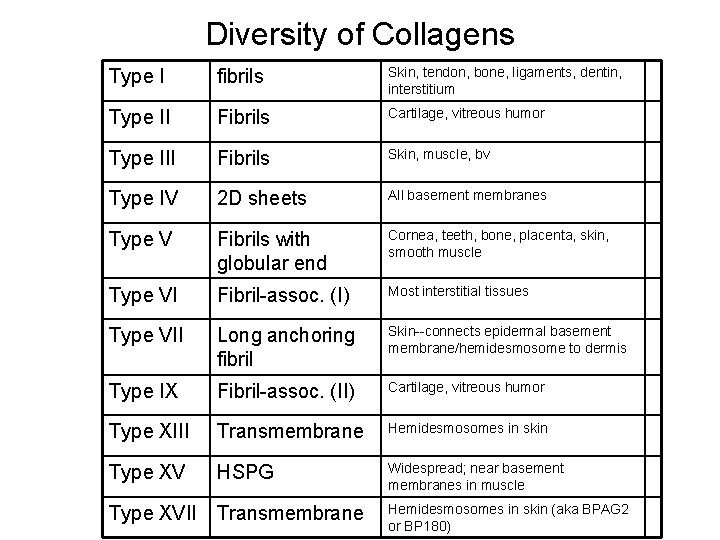
Diversity of Collagens Type I fibrils Skin, tendon, bone, ligaments, dentin, interstitium Type II Fibrils Cartilage, vitreous humor Type III Fibrils Skin, muscle, bv Type IV 2 D sheets All basement membranes Type V Fibrils with globular end Cornea, teeth, bone, placenta, skin, smooth muscle Type VI Fibril-assoc. (I) Most interstitial tissues Type VII Long anchoring fibril Skin--connects epidermal basement membrane/hemidesmosome to dermis Type IX Fibril-assoc. (II) Cartilage, vitreous humor Type XIII Transmembrane Hemidesmosomes in skin Type XV HSPG Widespread; near basement membranes in muscle Type XVII Transmembrane Hemidesmosomes in skin (aka BPAG 2 or BP 180)

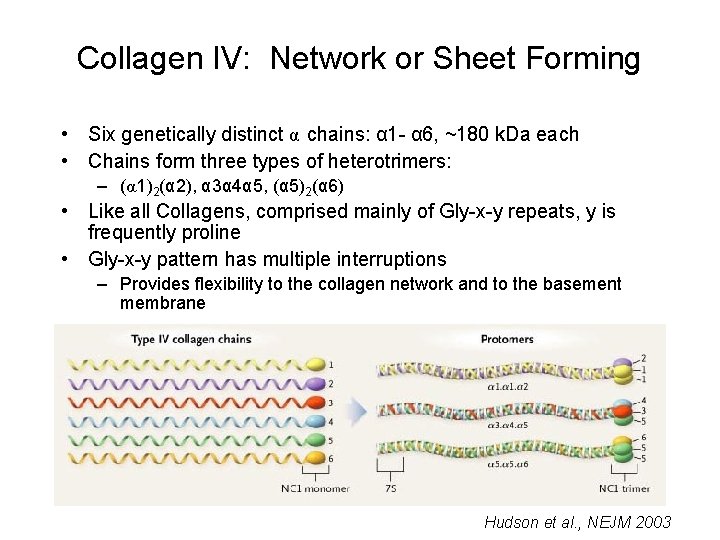
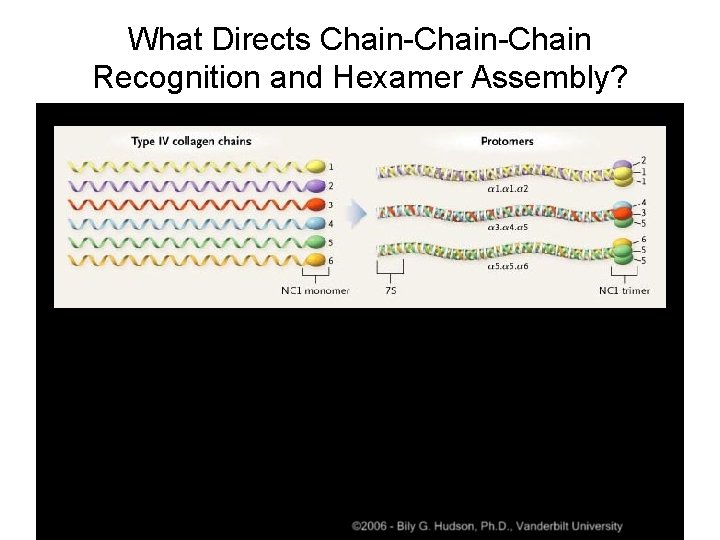
Collagen IV: Network or Sheet Forming • Six genetically distinct α chains: α 1 - α 6, ~180 k. Da each • Chains form three types of heterotrimers: – (α 1)2(α 2), α 3α 4α 5, (α 5)2(α 6) • Like all Collagens, comprised mainly of Gly-x-y repeats, y is frequently proline • Gly-x-y pattern has multiple interruptions – Provides flexibility to the collagen network and to the basement membrane Hudson et al. , NEJM 2003

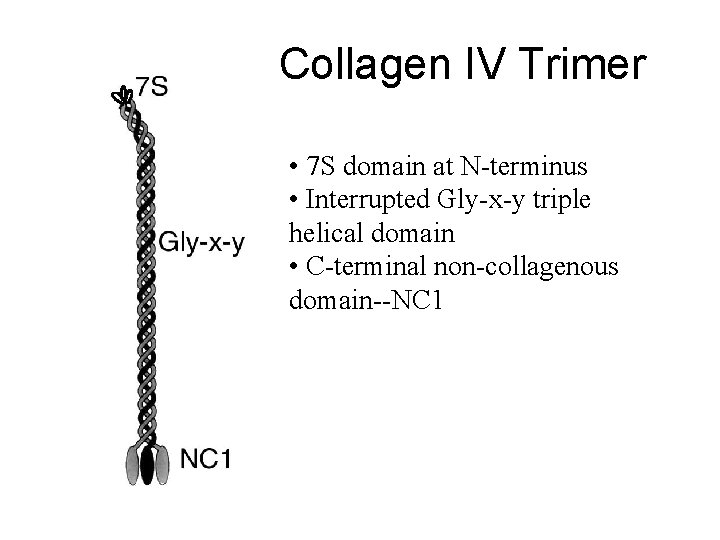
Collagen IV Trimer • 7 S domain at N-terminus • Interrupted Gly-x-y triple helical domain • C-terminal non-collagenous domain--NC 1

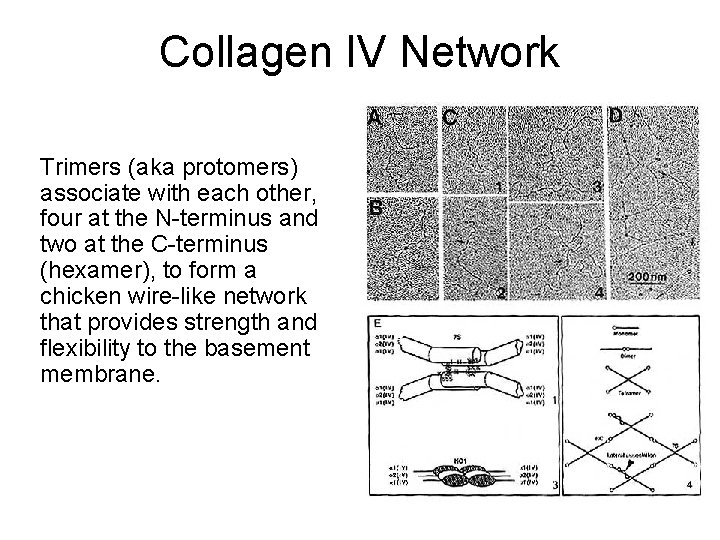
Collagen IV Network Trimers (aka protomers) associate with each other, four at the N-terminus and two at the C-terminus (hexamer), to form a chicken wire-like network that provides strength and flexibility to the basement membrane.

What Directs Chain-Chain Recognition and Hexamer Assembly?

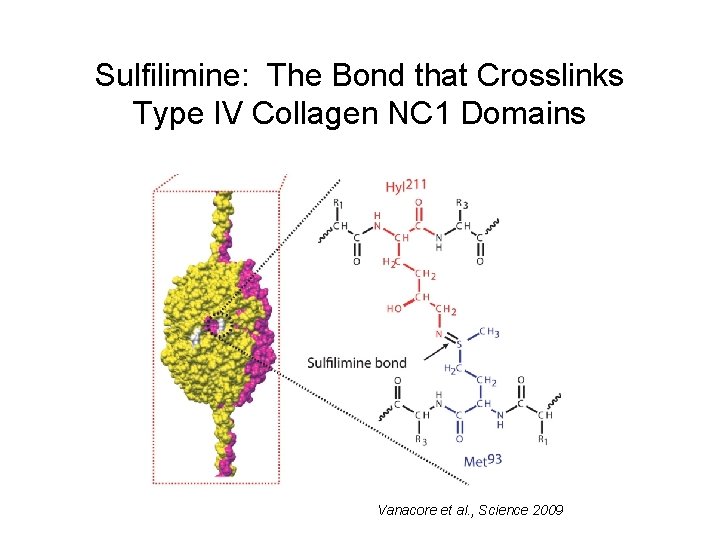
Sulfilimine: The Bond that Crosslinks Type IV Collagen NC 1 Domains Vanacore et al. , Science 2009

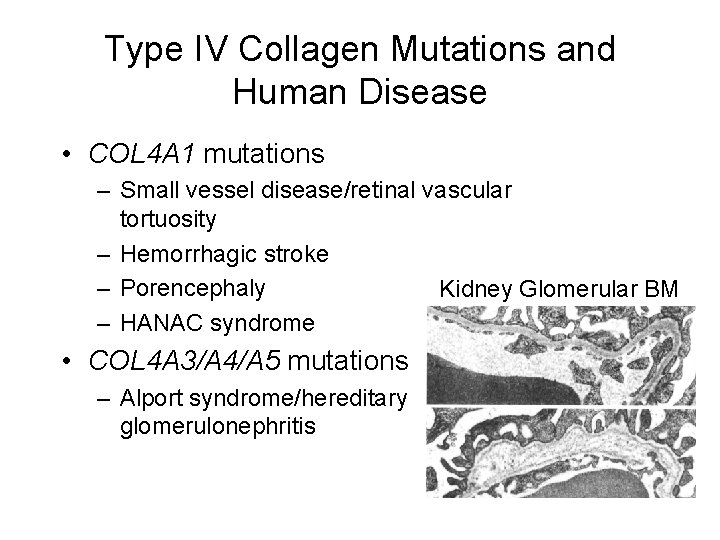
Type IV Collagen Mutations and Human Disease • COL 4 A 1 mutations – Small vessel disease/retinal vascular tortuosity – Hemorrhagic stroke – Porencephaly Kidney Glomerular BM – HANAC syndrome • COL 4 A 3/A 4/A 5 mutations – Alport syndrome/hereditary glomerulonephritis

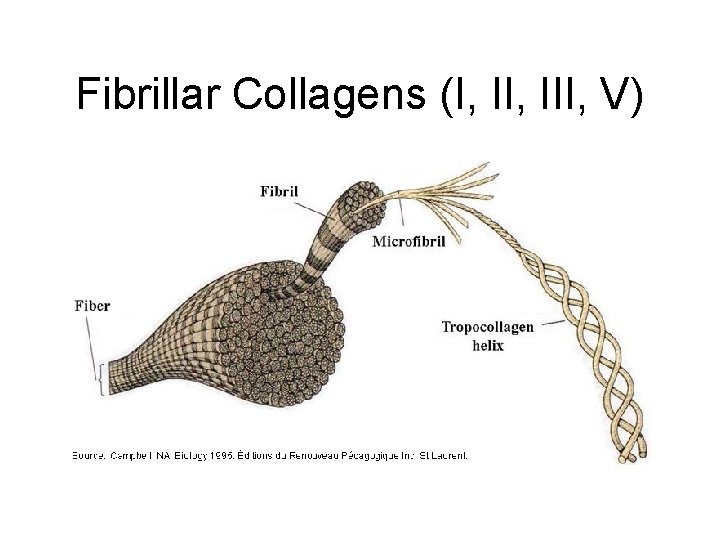
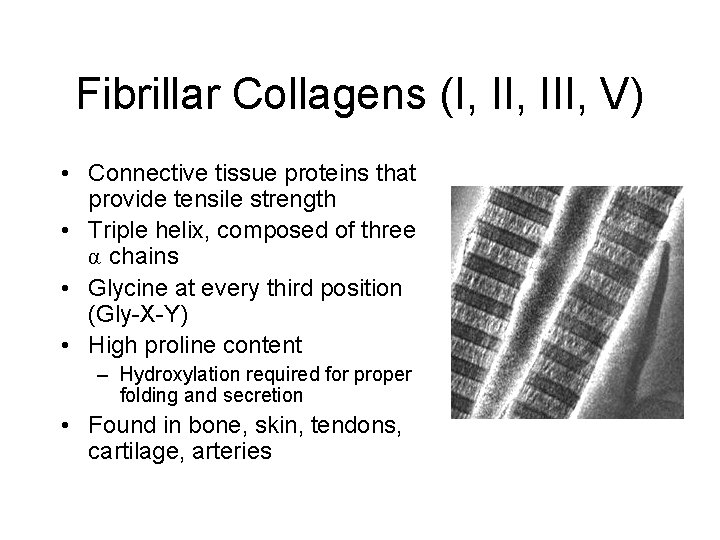
Fibrillar Collagens (I, III, V)

Fibrillar Collagens (I, III, V) • Connective tissue proteins that provide tensile strength • Triple helix, composed of three α chains • Glycine at every third position (Gly-X-Y) • High proline content – Hydroxylation required for proper folding and secretion • Found in bone, skin, tendons, cartilage, arteries

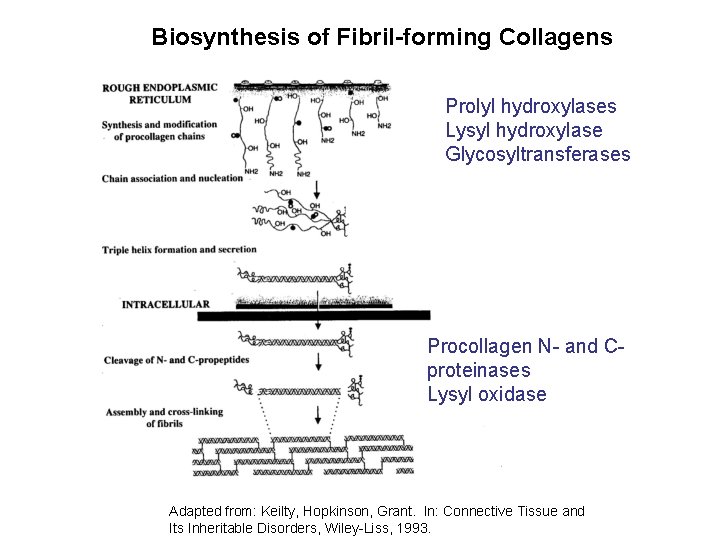
Biosynthesis of Fibril-forming Collagens Prolyl hydroxylases Lysyl hydroxylase Glycosyltransferases Procollagen N- and Cproteinases Lysyl oxidase Adapted from: Keilty, Hopkinson, Grant. In: Connective Tissue and Its Inheritable Disorders, Wiley-Liss, 1993.

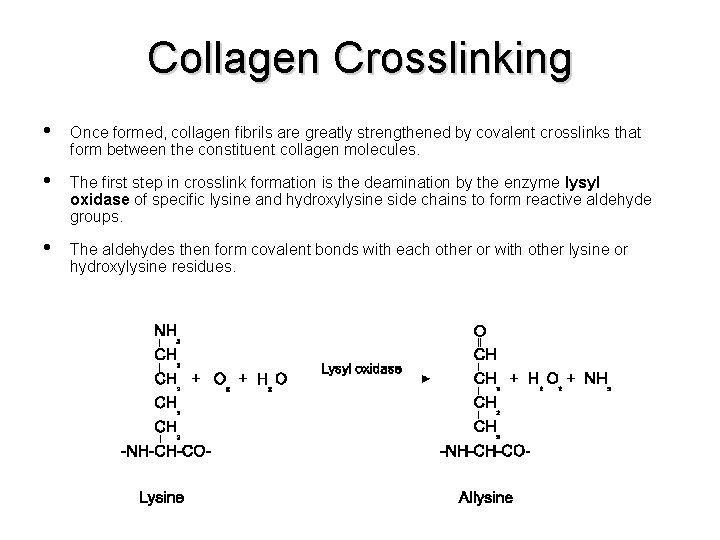
Collagen Crosslinking • Once formed, collagen fibrils are greatly strengthened by covalent crosslinks that form between the constituent collagen molecules. • The first step in crosslink formation is the deamination by the enzyme lysyl oxidase of specific lysine and hydroxylysine side chains to form reactive aldehyde groups. • The aldehydes then form covalent bonds with each other or with other lysine or hydroxylysine residues.

Collagen Crosslinking • If crosslinking is inhibited (Lysyl hydroxylase mutations; vitamin C deficiency), collagenous tissues become fragile, and structures such as skin, tendons, and blood vessels tend to tear. There also many bone manifestations of under-crosslinked collagen. • Hydroxylation of specific lysines governs the nature of the crosslink formed, which affects the biomechanical properties of the tissue. Collagen is especially highly crosslinked in the Achilles tendon, where tensile strength is crucial.

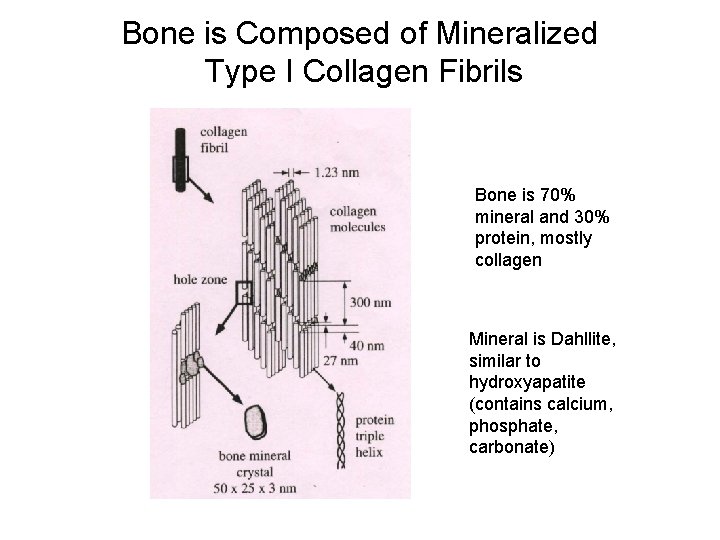
Bone is Composed of Mineralized Type I Collagen Fibrils Bone is 70% mineral and 30% protein, mostly collagen Mineral is Dahllite, similar to hydroxyapatite (contains calcium, phosphate, carbonate)

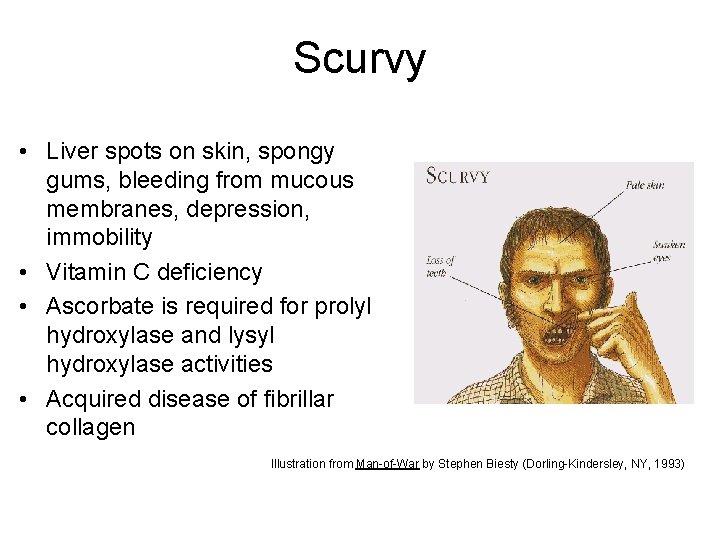
Scurvy • Liver spots on skin, spongy gums, bleeding from mucous membranes, depression, immobility • Vitamin C deficiency • Ascorbate is required for prolyl hydroxylase and lysyl hydroxylase activities • Acquired disease of fibrillar collagen Illustration from Man-of-War by Stephen Biesty (Dorling-Kindersley, NY, 1993)

Some Genetic Diseases of Collagen • Collagen I – Osteogenesis imperfecta – Ehlers-Danlos syndrome type VII • Collagen II – Multiple diseases of cartilage • Collagen III – Ehlers-Danlos syndrome type IV • Collagen IV – Alport syndrome, stroke, hemorrhage, porencephaly • Collagen VII – Dystrophic epidermolysis bullosa (skin blistering)

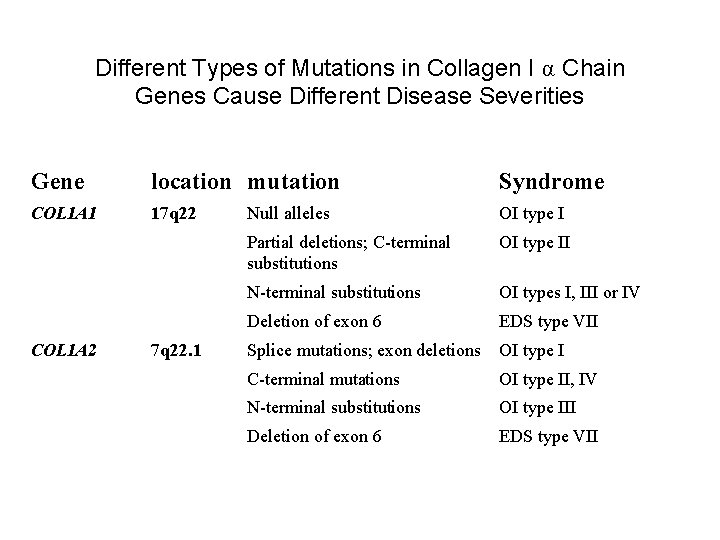
Different Types of Mutations in Collagen I α Chain Genes Cause Different Disease Severities Gene location mutation Syndrome COL 1 A 1 17 q 22 Null alleles OI type I Partial deletions; C-terminal substitutions OI type II N-terminal substitutions OI types I, III or IV Deletion of exon 6 EDS type VII COL 1 A 2 7 q 22. 1 Splice mutations; exon deletions OI type I C-terminal mutations OI type II, IV N-terminal substitutions OI type III Deletion of exon 6 EDS type VII

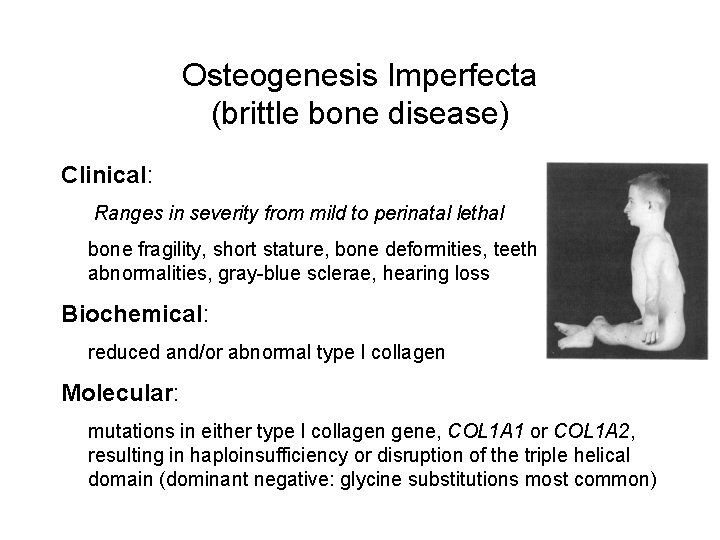
Osteogenesis Imperfecta (brittle bone disease) Clinical: Ranges in severity from mild to perinatal lethal bone fragility, short stature, bone deformities, teeth abnormalities, gray-blue sclerae, hearing loss Biochemical: reduced and/or abnormal type I collagen Molecular: mutations in either type I collagen gene, COL 1 A 1 or COL 1 A 2, resulting in haploinsufficiency or disruption of the triple helical domain (dominant negative: glycine substitutions most common)

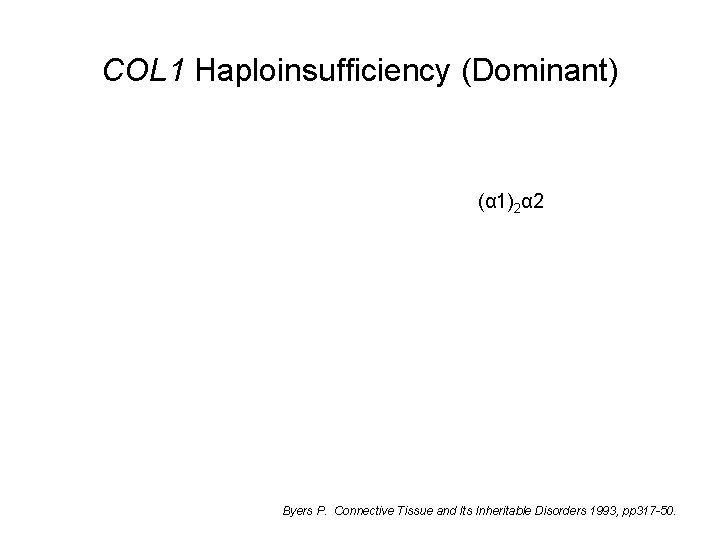
COL 1 Haploinsufficiency (Dominant) (α 1)2α 2 Byers P. Connective Tissue and Its Inheritable Disorders 1993, pp 317 -50.

Dominant Negative COL 1 Mutations ½ of the trimers are abnormal * Gly subst. in COL 4 A 2 * Gly subst. in COL 4 A 1 ¾ of the trimers are abnormal Byers P. Connective Tissue and Its Inheritable Disorders 1993, pp 317 -50.

Elastin and Elastic Fibers Exhibit Rubber-Like Properties • Physiological importance lies in the unique elastomeric properties of elastin. Found in tissues in which reversible extensibility or deformability are crucial, such as the major arterial vessels (esp. aorta), the lung and the skin. • Elastin is characterized by a high index of hydrophobicity (90% of all the amino acid residues are nonpolar). One-third of the amino acid residues are glycine with a preponderance of the nonpolar amino acids Ala, Val, Leu, and Ile. As in collagen, oneninth of the residues are proline (but with very little hydroxylation). • Early in development, the elastic fibers consists of microfibrils, which define fiber location and morphology. Over time, tropoelastin accumulates within the bed of microfibrils.

Elastic Fiber Biogenesis • • Elastic fibers are very complex, difficult to repair structures There are two morphologically distinguishable components – – • Microfibrils Elastin Assembly follows a well-defined sequence of events: 1. Assembly of microfibrils 2. Association of tropoelastin aggregates with microfibrils 3. Crosslinking of tropoelastins with each other by lysyl oxidase to form polymers Shifren and Mecham, 2006

Major steps underlying the assembly of microfibrils and elastic fibers Ramirez, F. et al. Physiol. Genomics 19: 151 -154 2004; doi: 10. 1152/physiolgenomics. 00092. 2004 Copyright © 2004 American Physiological Society

Microfibril Components: ~30 • Fibrillin--three forms • Microfibril-associated glycoproteins (MAGPs)--two forms • Latent TGFβ Binding Proteins (LTBPs)--four forms • Proteoglycans, MFAPs, Fibulins, Emilins, Collagens, Decorin, et al.

Fibrillins • Large glycoproteins (~350 k. Da) whose primary structures are dominated by cb. EGF domains that, in the presence of Ca 2+, adopt a rodlike structure • Limited intracellular assembly may occur, but microfibril assembly initiates at the cell surface after secretion, perhaps with the help of cellular receptors

Marfan Syndrome • Caused by dominant Fibrillin 1 (FBN 1) mutations – Haploinsufficiency is the culprit • Skeletal, ocular, and cardiovascular defects • Deficiency of elastinassociated microfibrils • Syndrome may result from alterations in TGFβ signaling, rather than purely structural changes in microfibrils

Latent TGFβ Binding Proteins • Members of the fibrillin superfamily • Maintain TGFβ in the inactive state by forming the “large latent complex”

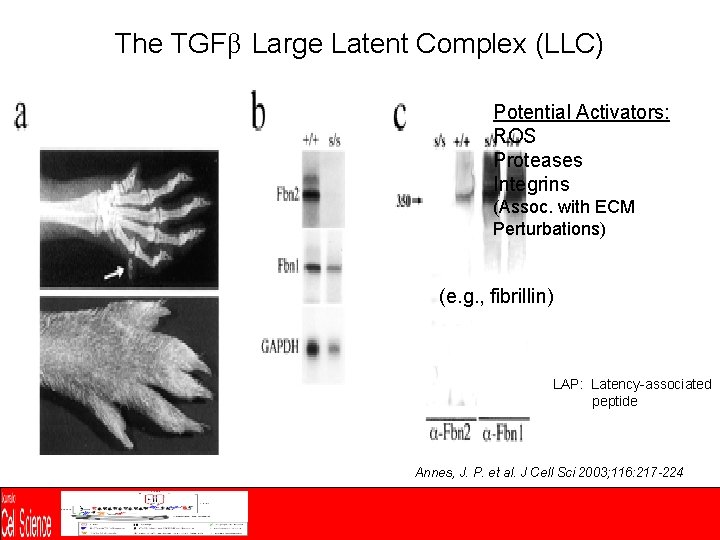
The TGFβ Large Latent Complex (LLC) Potential Activators: ROS Proteases Integrins (Assoc. with ECM Perturbations) (e. g. , fibrillin) LAP: Latency-associated peptide Annes, J. P. et al. J Cell Sci 2003; 116: 217 -224

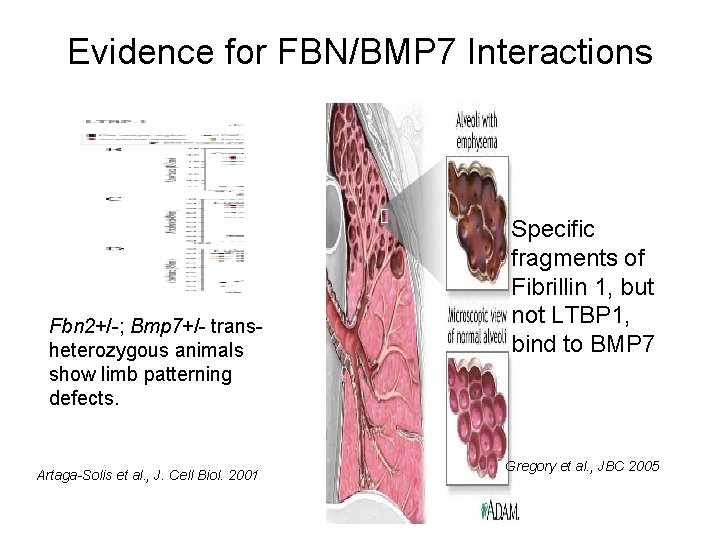
Evidence for FBN/BMP 7 Interactions Fbn 2+/-; Bmp 7+/- transheterozygous animals show limb patterning defects. Artaga-Solis et al. , J. Cell Biol. 2001 Specific fragments of Fibrillin 1, but not LTBP 1, bind to BMP 7 Gregory et al. , JBC 2005

Elastic Fiber Biogenesis • • Elastic fibers are very complex, difficult to repair structures There are two morphologically distinguishable components – – • Microfibrils Elastin Assembly follows a well-defined sequence of events: 1. Assembly of microfibrils 2. Association of tropoelastin aggregates with microfibrils 3. Crosslinking of tropoelastins with each other by lysyl oxidase to form polymers Shifren and Mecham, 2006

Emphysema • Damage to the lung air sacs (alveoli) that affects breathing • Macrophages induced to “ingest” particles in smoke also secrete proteases that degrade elastic fibers • Loss of lung elasticity makes exhalation difficult • Increased alveolar size reduces the surface area for gas exchange
 Wohl clinic
Wohl clinic Splen
Splen Matriz extracelular
Matriz extracelular Andy wohl
Andy wohl Wohl library
Wohl library Gesunde schule projekt
Gesunde schule projekt Robert wohl
Robert wohl Extracellular fluid composition
Extracellular fluid composition Extracellular fluid
Extracellular fluid Interstitial fluid vs extracellular fluid
Interstitial fluid vs extracellular fluid Deuteromycotes
Deuteromycotes Body fluids
Body fluids Extracellular signal regulated kinase
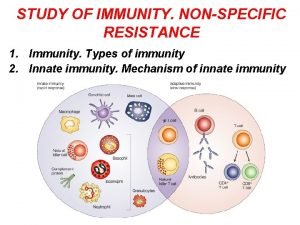
Extracellular signal regulated kinase Non specific innate immunity
Non specific innate immunity Fluid sf
Fluid sf Extracellular digestion
Extracellular digestion Interstitial fluid vs extracellular fluid
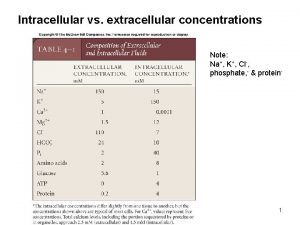
Interstitial fluid vs extracellular fluid Cl extracellular concentration
Cl extracellular concentration Fungsi membran sel
Fungsi membran sel Intracellular and extracellular
Intracellular and extracellular Outline two roles of extracellular components
Outline two roles of extracellular components Extracellular fluid and interstitial fluid
Extracellular fluid and interstitial fluid Major intra and extracellular electrolytes
Major intra and extracellular electrolytes Nature reviews immunology
Nature reviews immunology Transcellular fluid
Transcellular fluid Extracellular signal
Extracellular signal Max miner algorithm
Max miner algorithm Janice miner holden
Janice miner holden The alchemist why does the lake weep for narcissus
The alchemist why does the lake weep for narcissus Kris miner
Kris miner Data mining confluence of multiple disciplines
Data mining confluence of multiple disciplines Kenora daily miner and news
Kenora daily miner and news Finex miner
Finex miner Kyohei okumura
Kyohei okumura Sander leemans
Sander leemans Utep minermall
Utep minermall Meni miner
Meni miner Miner arc
Miner arc Kenora daily miner
Kenora daily miner Charm box nacirema
Charm box nacirema Holly leaf miner
Holly leaf miner N'vivo
N'vivo Ibm intelligent miner
Ibm intelligent miner New miner training record/certificate
New miner training record/certificate Helvetius elias fulcanelli geber
Helvetius elias fulcanelli geber Exam miner 42
Exam miner 42 Newly hired experienced miner training
Newly hired experienced miner training Pjm data miner 2
Pjm data miner 2 Tư thế worms-breton
Tư thế worms-breton ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Tư thế ngồi viết
Tư thế ngồi viết Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Bổ thể
Bổ thể Tư thế ngồi viết
Tư thế ngồi viết V cc cc
V cc cc Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chúa yêu trần thế
Chúa yêu trần thế Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Cong thức tính động năng
Cong thức tính động năng Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể