The Evolution of Colorectal Cancer Screening Survival with

















- Slides: 50

The Evolution of Colorectal Cancer Screening Survival with the FIT-Test Dr. Jan Owen & Dr. Brian Yan May 9, 2018

Faculty/Presenter Disclosure • Faculty: Dr. Jan Owen and Dr. Brian Yan • Relationships with commercial interests: o o Grants/Research Support: None Speakers Bureau/Honoraria: None Consulting Fees: None Other: Employees of Cancer Care Ontario • Potential for conflict(s) of interest: o None • All information provided in presentation has been provided by Cancer Care Ontario.

Learning Objectives 1. Introduce a new test for colorectal cancer screening: the Fecal Immunochemical Test (FIT) 2. Examine evidence to support FIT (instead of colonoscopy) for screening in persons at average risk for colorectal cancer 3. Discuss the expected outcomes of a positive FIT colonoscopy 4. Describe changes to the Colon. Cancer. Check program 5. Discuss primary care providers’ role in FIT implementation in Ontario 3

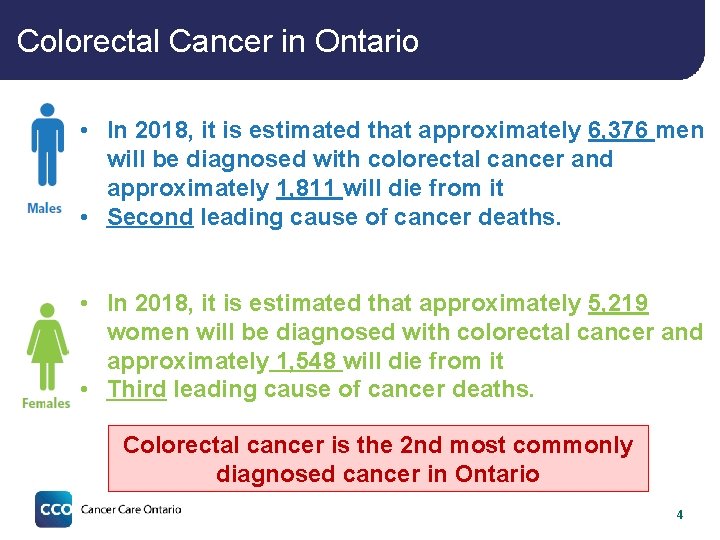
Colorectal Cancer in Ontario • In 2018, it is estimated that approximately 6, 376 men will be diagnosed with colorectal cancer and approximately 1, 811 will die from it • Second leading cause of cancer deaths. • In 2018, it is estimated that approximately 5, 219 women will be diagnosed with colorectal cancer and approximately 1, 548 will die from it • Third leading cause of cancer deaths. Colorectal cancer is the 2 nd most commonly diagnosed cancer in Ontario 4

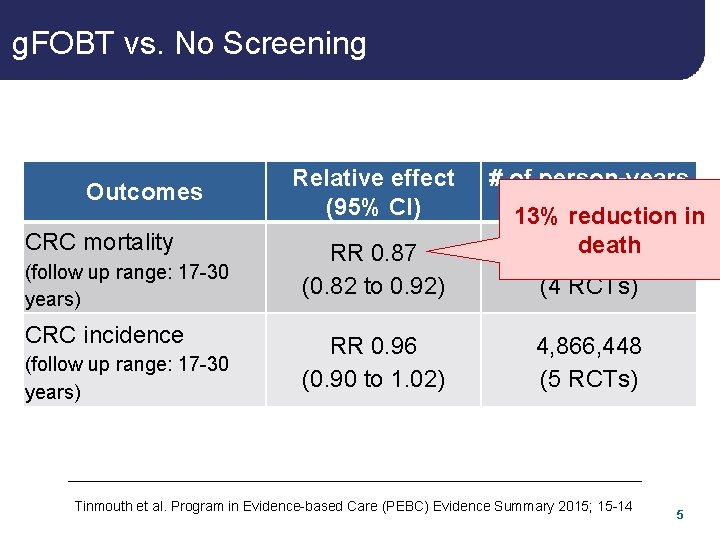
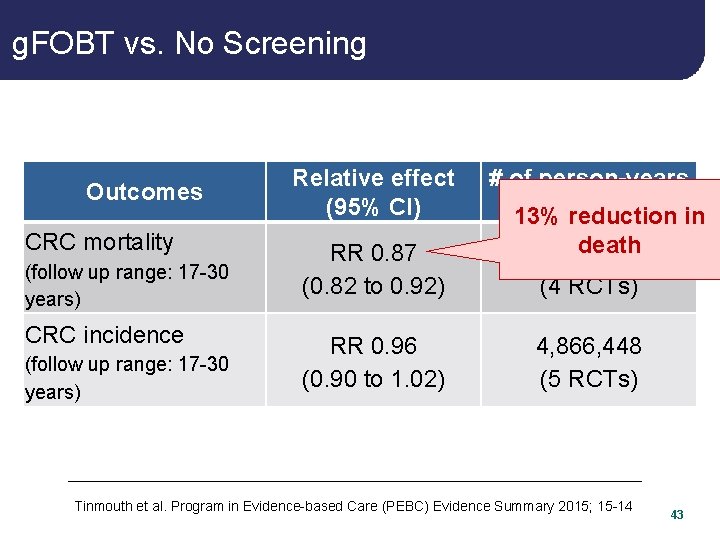
g. FOBT vs. No Screening Outcomes CRC mortality (follow up range: 17 -30 years) CRC incidence (follow up range: 17 -30 years) Relative effect (95% CI) # of person-years (# of reduction studies) in 13% RR 0. 87 (0. 82 to 0. 92) death 5, 344, 100 (4 RCTs) RR 0. 96 (0. 90 to 1. 02) 4, 866, 448 (5 RCTs) Tinmouth et al. Program in Evidence-based Care (PEBC) Evidence Summary 2015; 15 -14 5

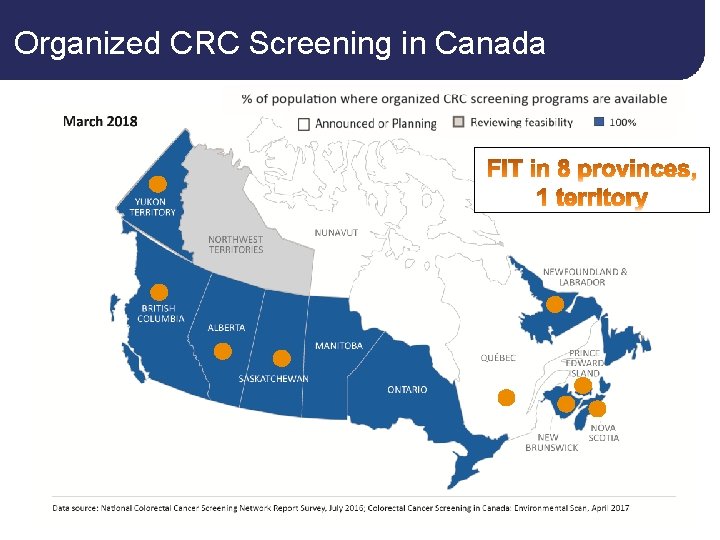
Organized CRC Screening in Canada 6

CCC is planning to implement FIT as the recommended test for people at average risk of CRC 7

What is FIT? • Fecal immunochemical test • At-home CRC screening test • One sample • Tube designed for easy sampling • Automated test processing at laboratory • Quantitative result 8

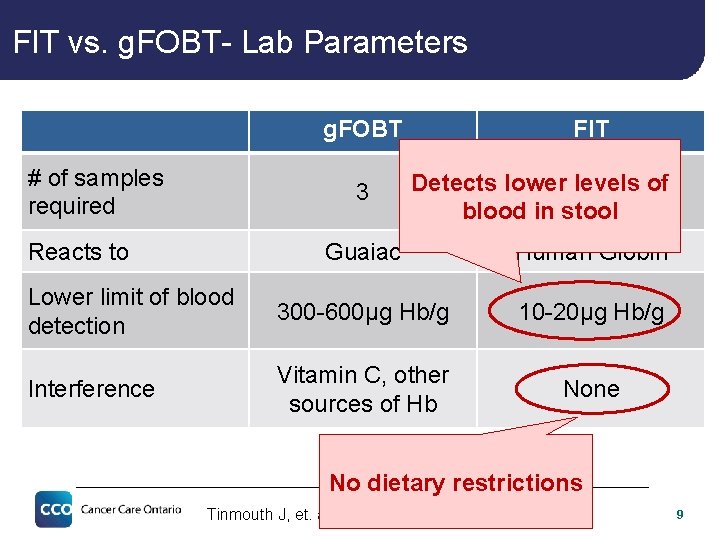
FIT vs. g. FOBT- Lab Parameters g. FOBT # of samples required 3 Reacts to FIT Detects lower levels of 1 blood in stool Guaiac Human Globin Lower limit of blood detection 300 -600µg Hb/g 10 -20µg Hb/g Interference Vitamin C, other sources of Hb None No dietary restrictions Tinmouth J, et. al. Gut. 2015 Aug; 64(8): 1327 -37. 9

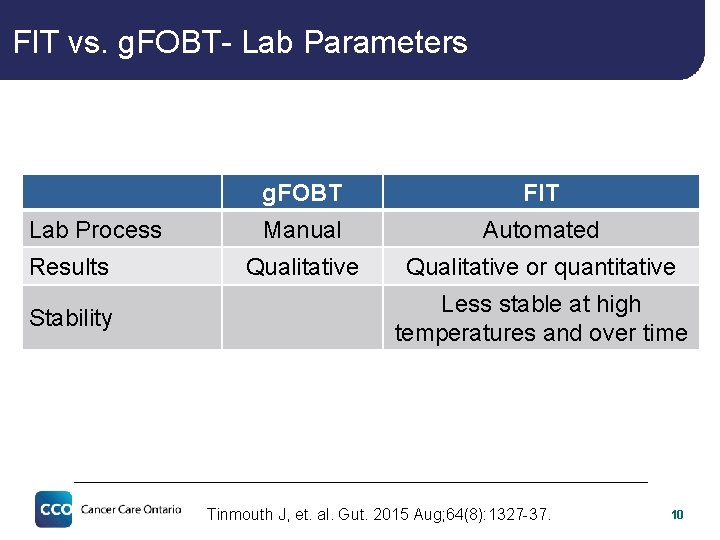
FIT vs. g. FOBT- Lab Parameters Lab Process Results Stability g. FOBT FIT Manual Automated Qualitative or quantitative Less stable at high temperatures and over time Tinmouth J, et. al. Gut. 2015 Aug; 64(8): 1327 -37. 10

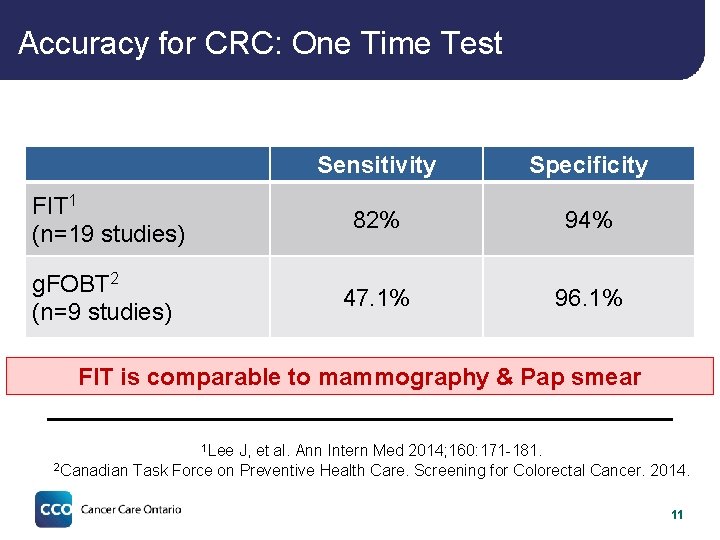
Accuracy for CRC: One Time Test Sensitivity Specificity FIT 1 (n=19 studies) 82% 94% g. FOBT 2 (n=9 studies) 47. 1% 96. 1% FIT is comparable to mammography & Pap smear 1 Lee 2 Canadian J, et al. Ann Intern Med 2014; 160: 171 -181. Task Force on Preventive Health Care. Screening for Colorectal Cancer. 2014. 11

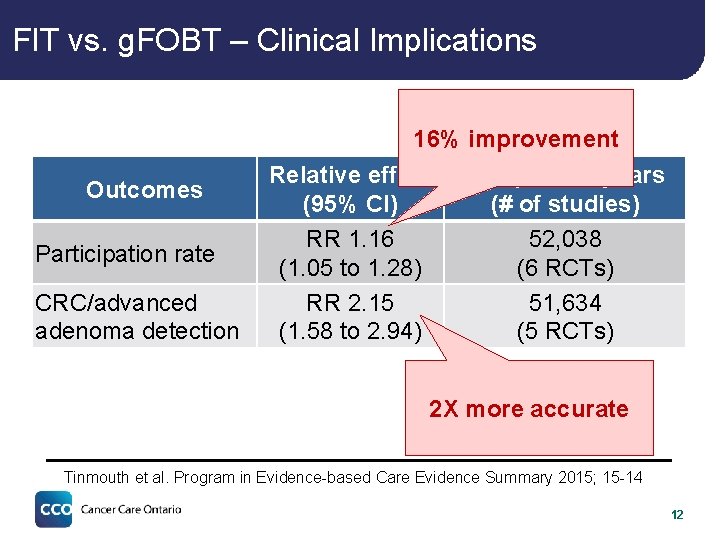
FIT vs. g. FOBT – Clinical Implications 16% improvement Outcomes Participation rate CRC/advanced adenoma detection Relative effect (95% CI) RR 1. 16 (1. 05 to 1. 28) RR 2. 15 (1. 58 to 2. 94) # of person-years (# of studies) 52, 038 (6 RCTs) 51, 634 (5 RCTs) 2 X more accurate Tinmouth et al. Program in Evidence-based Care Evidence Summary 2015; 15 -14 12

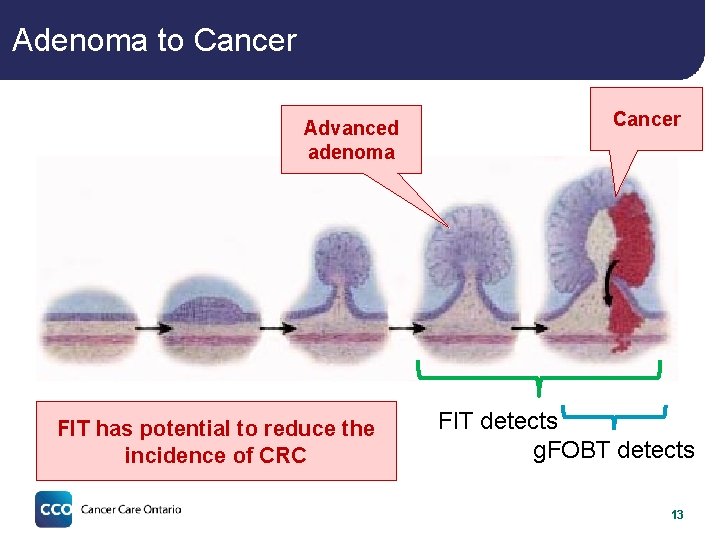
Adenoma to Cancer Advanced adenoma FIT has potential to reduce the incidence of CRC Cancer FIT detects g. FOBT detects 13

FIT vs. Colonoscopy for Average Risk Screening: The Evidence 14

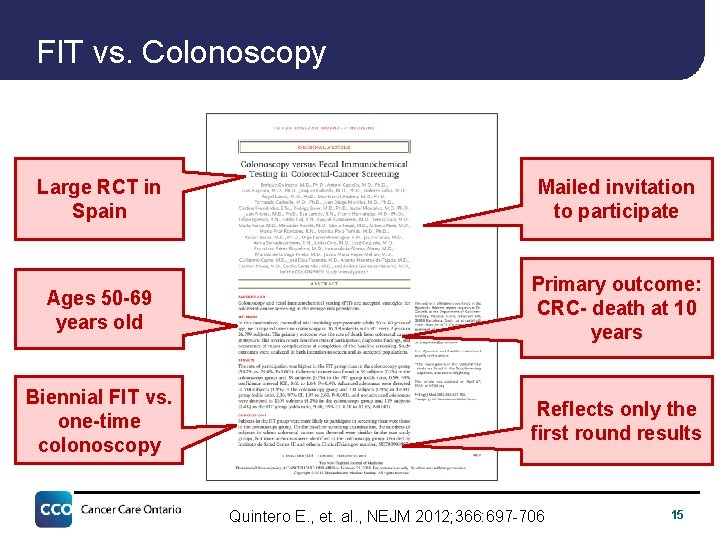
FIT vs. Colonoscopy Large RCT in Spain Mailed invitation to participate Ages 50 -69 years old Primary outcome: CRC- death at 10 years Biennial FIT vs. one-time colonoscopy Reflects only the first round results Quintero E. , et. al. , NEJM 2012; 366: 697 -706 15

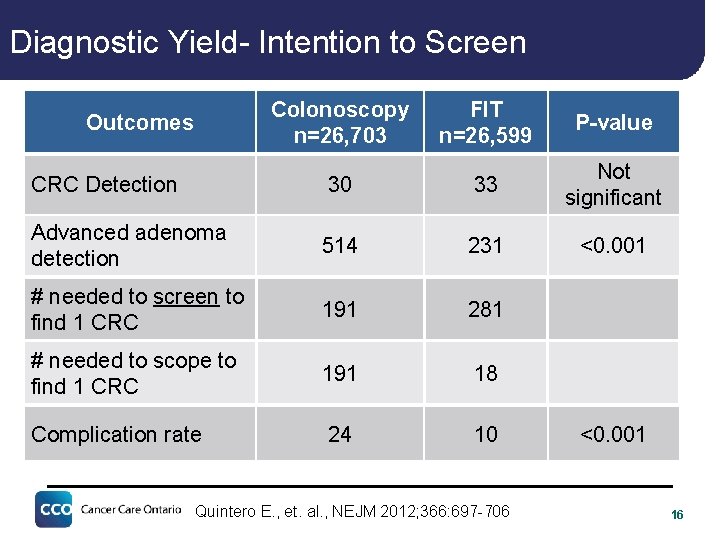
Diagnostic Yield- Intention to Screen Colonoscopy n=26, 703 Outcomes FIT n=26, 599 P-value CRC Detection 30 33 Not significant Advanced adenoma detection 514 231 <0. 001 # needed to screen to find 1 CRC 191 281 # needed to scope to find 1 CRC 191 18 Complication rate 24 10 Quintero E. , et. al. , NEJM 2012; 366: 697 -706 <0. 001 16

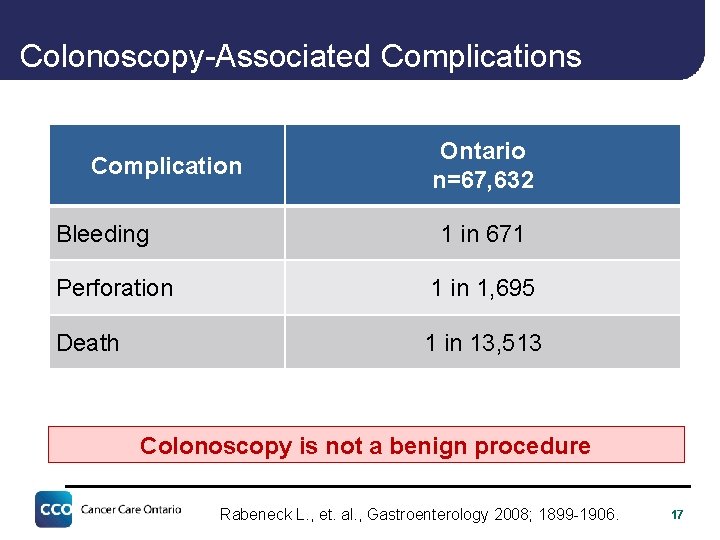
Colonoscopy-Associated Complications Complication Bleeding Ontario n=67, 632 1 in 671 Perforation 1 in 1, 695 Death 1 in 13, 513 Colonoscopy is not a benign procedure Rabeneck L. , et. al. , Gastroenterology 2008; 1899 -1906. 17

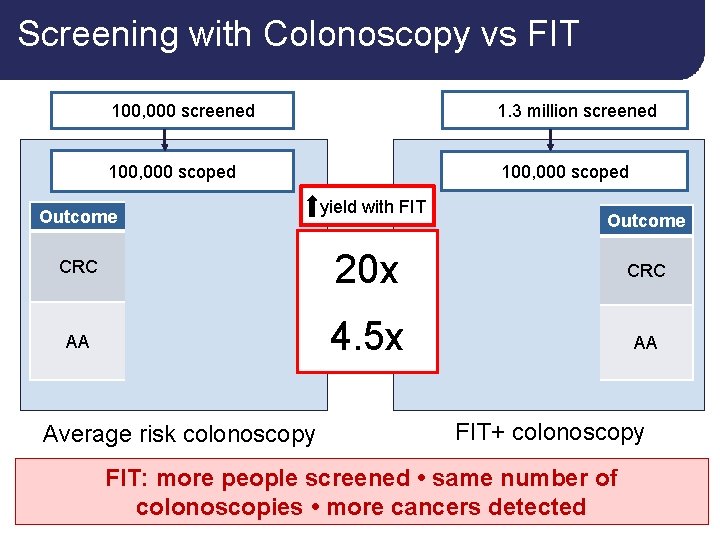
Screening with Colonoscopy vs FIT 100, 000 screened 1. 3 million screened 100, 000 scoped Outcome PPV Yield CRC 0. 4% 400 AA 10% 10, 000 Average risk colonoscopy yield with FIT Yield PPV Outcome 20 x 8, 000 8% CRC 4. 5 x 45, 000 45% AA FIT+ colonoscopy FIT: more people screened • same number of colonoscopies • more cancers detected 18

FIT vs. Colonoscopy for Average Risk Screening: FIT Experience in Alberta 19

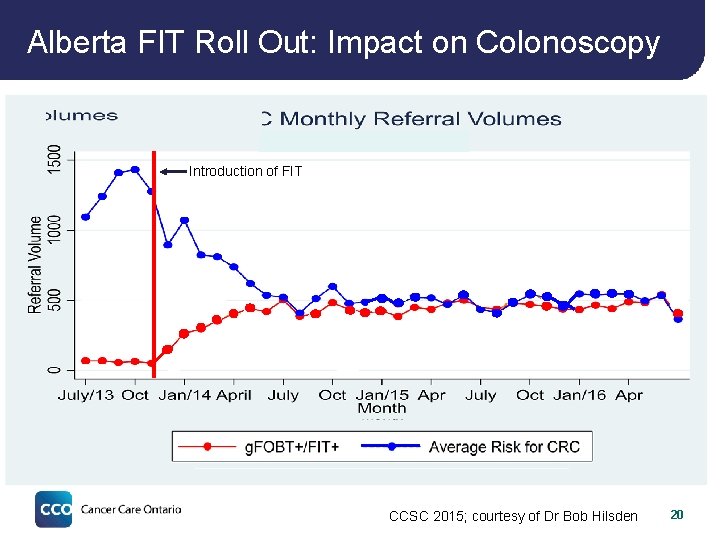
Alberta FIT Roll Out: Impact on Colonoscopy Introduction of FIT CCSC 2015; courtesy of Dr Bob Hilsden 20

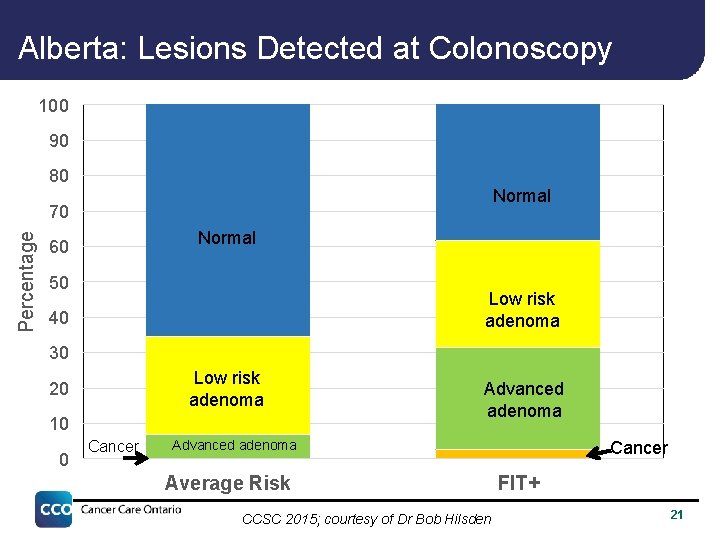
Alberta: Lesions Detected at Colonoscopy 100 90 80 Normal Percentage 70 Normal 60 50 Low risk adenoma 40 30 Low risk adenoma 20 10 0 Cancer Advanced adenoma Average Risk CCSC 2015; courtesy of Dr Bob Hilsden Cancer FIT+ 21

FIT vs. Colonoscopy – Take Home • FIT is preferred by patients • FIT is as good as colonoscopy for detecting CRC in average risk patients • FIT is safer than colonoscopy • FIT-positive colonoscopy is high yield → colonoscopy used in persons most likely to benefit FIT Better risk – benefit ratio of screening • The CCC program does not recommend screening with colonoscopy for average risk patients 22

Transition to FIT 23

Who Should be Screened with FIT? The Colon. Cancer. Check eligibility criteria for individuals: • Ages 50 -74 at average risk • No first degree relative diagnosed with colorectal cancer • Asymptomatic • No screening with g. FOBT or FIT in the past two years • No screening with colonoscopy or flexible sigmoidoscopy in the past 10 years • Valid Ontario Health Insurance Plan number Eligibility criteria have not changed 24

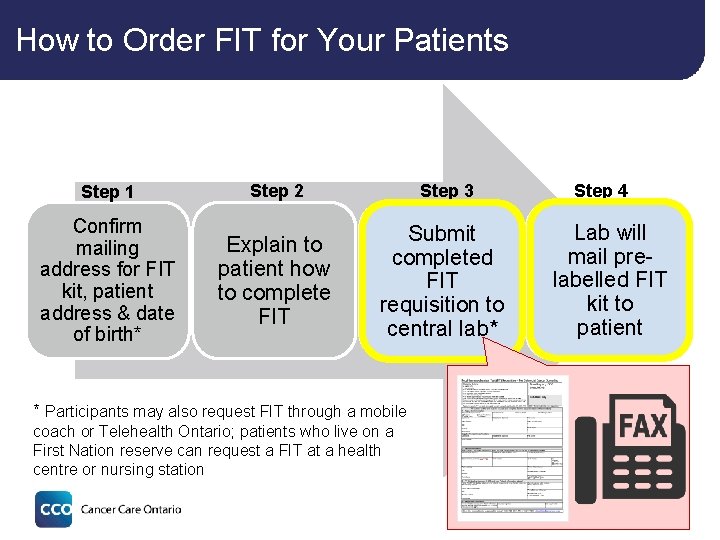
How to Order FIT for Your Patients Step 1 Confirm mailing address for FIT kit, patient address & date of birth* Step 3 Step 2 Explain to patient how to complete FIT Submit completed FIT requisition to requisition central lab* lab Step 4 Lab will mail prepre-labelled FIT FITkitkit toto patient * Participants may also request FIT through a mobile coach or Telehealth Ontario; patients who live on a First Nation reserve can request a FIT at a health centre or nursing station 25

Requisition Changes Regular lab requisition cannot be used to request CCC program FIT (or g. FOBT) 26

New FIT Requisition New FIT requisition 27

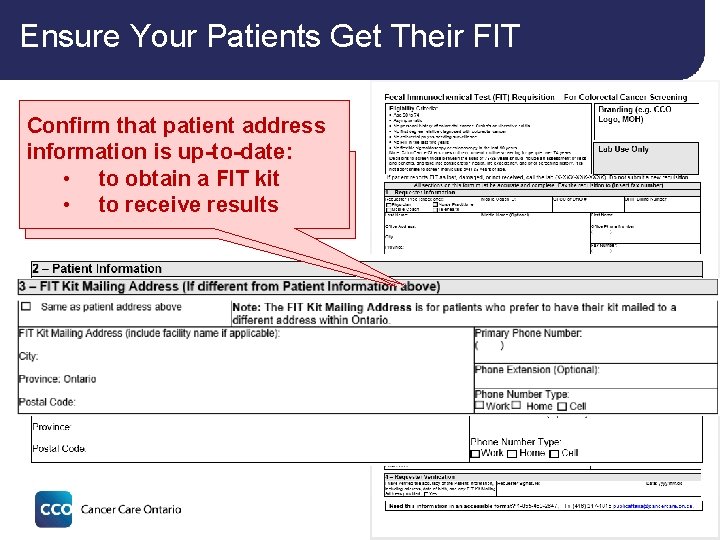
Ensure Your Patients Get Their FIT Confirm that patient address information is up-to-date: • to obtain FIT kit Alternate FIT kitadelivery • to receive results option 28

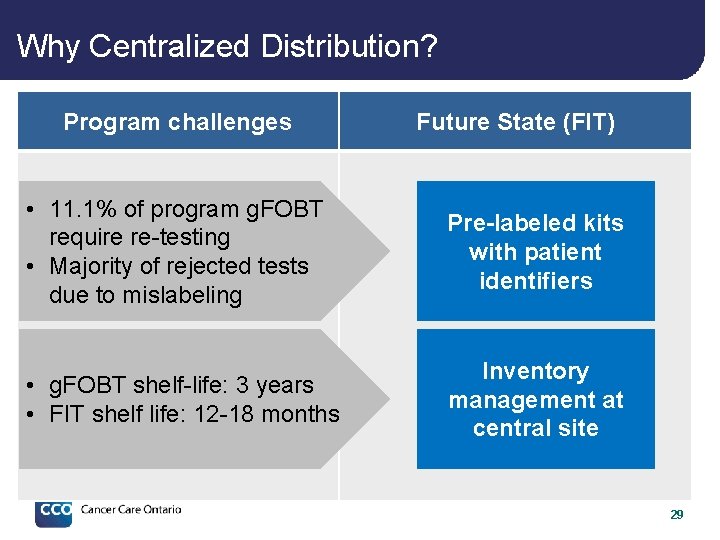
Why Centralized Distribution? Program challenges Future State (FIT) • 11. 1% of program g. FOBT require re-testing • Majority of rejected tests due to mislabeling Pre-labeled kits with patient identifiers • g. FOBT shelf-life: 3 years • FIT shelf life: 12 -18 months Inventory management at central site 29

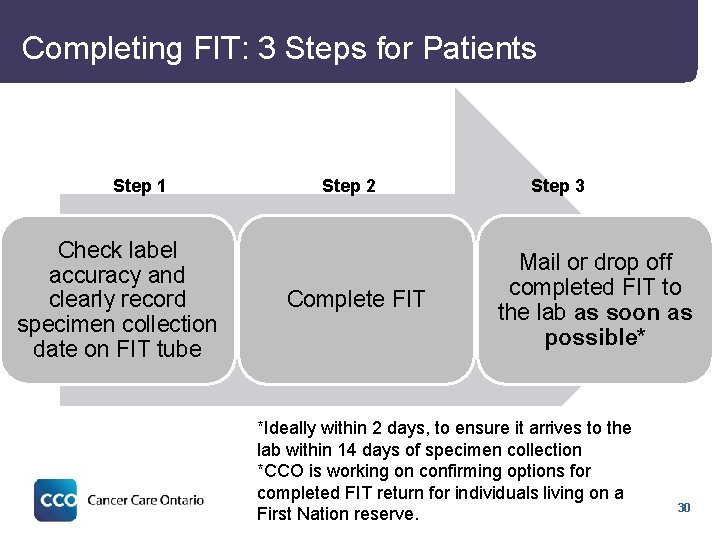
Completing FIT: 3 Steps for Patients Step 1 Check label accuracy and clearly record specimen collection date on FIT tube Step 2 Complete FIT Step 3 Mail or drop off completed FIT to the lab as soon as possible* *Ideally within 2 days, to ensure it arrives to the lab within 14 days of specimen collection *CCO is working on confirming options for completed FIT return for individuals living on a First Nation reserve. 30

Multiple Options for FIT Return • Mail - Regular mail - Xpresspost included for some areas • Drop off at lab specimen collection centres • CCO is working on confirming options for completed FIT return for individuals living on a First Nation reserve 31

Supporting Patients • User tested, patientfriendly FIT materials are being developed, including FIT instructions that use more visuals than words T F A R D • Patients will continue to receive CCC correspondence 32

How to Manage Patients with an Abnormal FIT 33

Case study #1 Anna is a 64 year old woman who has recently completed a Fecal Immunochemical Test (FIT). When her FIT result comes back as abnormal (positive), Anna calls you and mentions that she completed her FIT just one day after having a tooth removed by her dentist. Anna would like to repeat the FIT. What should you do and why? 34

Case study #1 What should you do and why? a) Complete another FIT requisition for Anna b) Refer Anna for flexible sigmoidoscopy c) Have Anna come for an in-office g. FOBT d) Counsel Anna on the importance of a followup colonoscopy and refer her promptly for colonoscopy e) None of the above 35

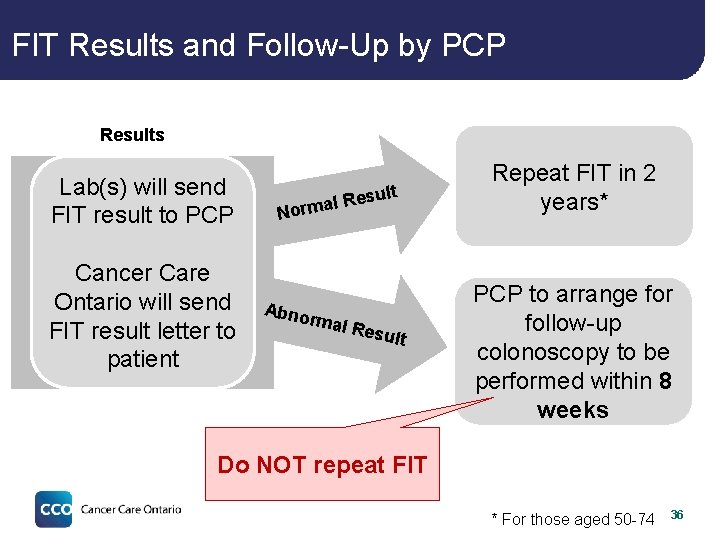
FIT Results and Follow-Up by PCP Results Lab(s) will send FIT result to PCP Cancer Care Ontario will send FIT result letter to patient ult Res l a m r No Abno rmal Resu lt Repeat FIT in 2 years* PCP to arrange for follow-up colonoscopy to be performed within 8 weeks Do NOT repeat FIT * For those aged 50 -74 36

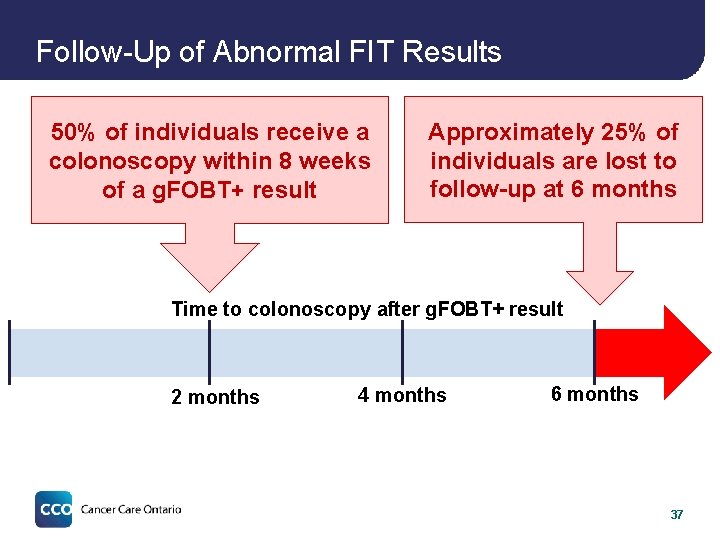
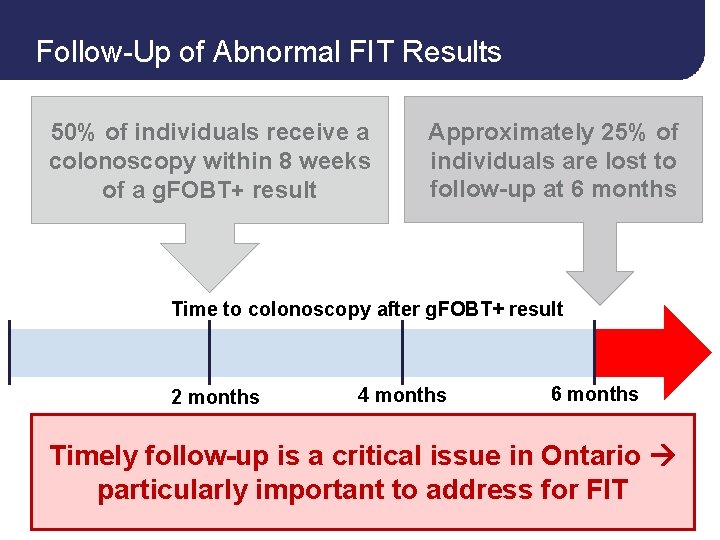
Follow-Up of Abnormal FIT Results 50% of individuals receive a colonoscopy within 8 weeks of a g. FOBT+ result Approximately 25% of individuals are lost to follow-up at 6 months Time to colonoscopy after g. FOBT+ result 2 months 4 months 6 months 37

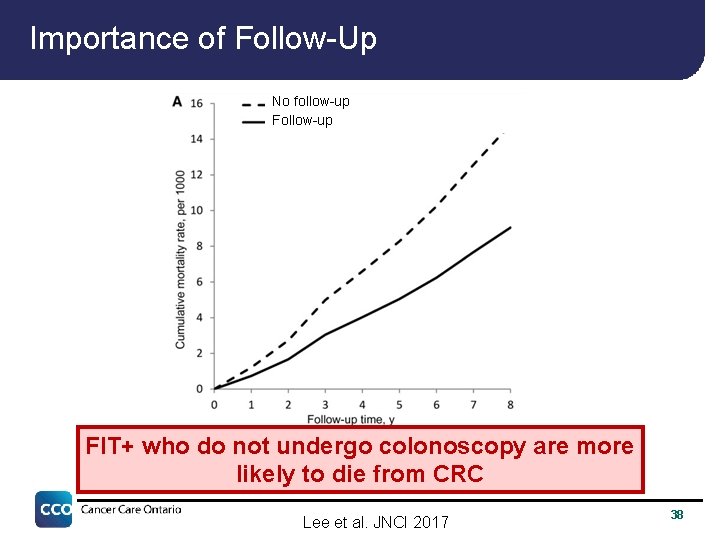
Importance of Follow-Up No follow-up FIT+ who do not undergo colonoscopy are more likely to die from CRC Lee et al. JNCI 2017 38

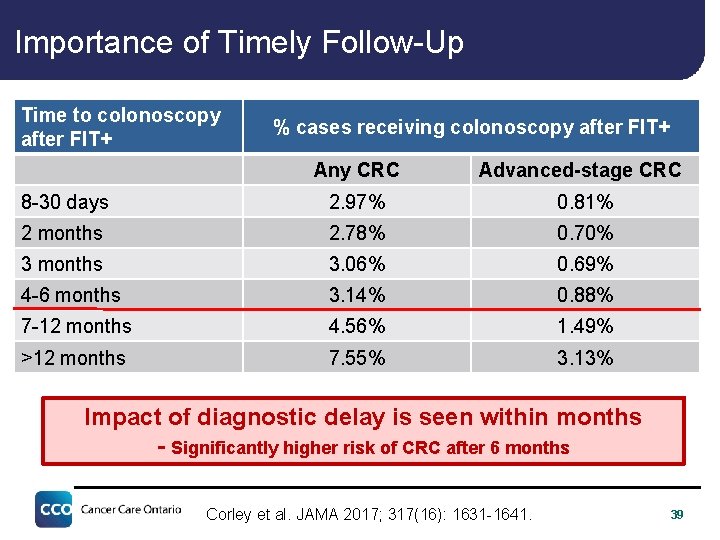
Importance of Timely Follow-Up Time to colonoscopy after FIT+ % cases receiving colonoscopy after FIT+ Any CRC Advanced-stage CRC 8 -30 days 2. 97% 0. 81% 2 months 2. 78% 0. 70% 3 months 3. 06% 0. 69% 4 -6 months 3. 14% 0. 88% 7 -12 months 4. 56% 1. 49% >12 months 7. 55% 3. 13% Impact of diagnostic delay is seen within months - Significantly higher risk of CRC after 6 months Corley et al. JAMA 2017; 317(16): 1631 -1641. 39

Follow-Up of Abnormal FIT Results 50% of individuals receive a colonoscopy within 8 weeks of a g. FOBT+ result Approximately 25% of individuals are lost to follow-up at 6 months Time to colonoscopy after g. FOBT+ result 2 months 4 months 6 months Timely follow-up is a critical issue in Ontario particularly important to address for FIT 40

Case Study #2 Your 53 year old patient is due for g. FOBT screening in June 2018. John, who has no family history of colorectal cancer, has heard about the Fecal Immunochemical Test (FIT) and would like to either wait for the introduction of FIT or complete both the g. FOBT and FIT in 2018. What would be the appropriate response to John?

Case Study #2 What would be the appropriate response to John? a) Tell John he can delay screening until FIT is available later in the year b) Tell John he can complete both screening tests c) Tell John he should not delay screening and should complete the g. FOBT d) Tell John that the FIT is not as good as the g. FOBT at detecting colorectal cancer e) Both c and d

g. FOBT vs. No Screening Outcomes CRC mortality (follow up range: 17 -30 years) CRC incidence (follow up range: 17 -30 years) Relative effect (95% CI) # of person-years (# of reduction studies) in 13% RR 0. 87 (0. 82 to 0. 92) death 5, 344, 100 (4 RCTs) RR 0. 96 (0. 90 to 1. 02) 4, 866, 448 (5 RCTs) Tinmouth et al. Program in Evidence-based Care (PEBC) Evidence Summary 2015; 15 -14 43

When will FIT be Available in Ontario? • Until further notice: g. FOBT remains the recommended CRC screening test in Ontario • We are actively working towards FIT: coming soon! D E N U T Y A ST Funding Quality assurance Communications IT Laboratories Program design 44

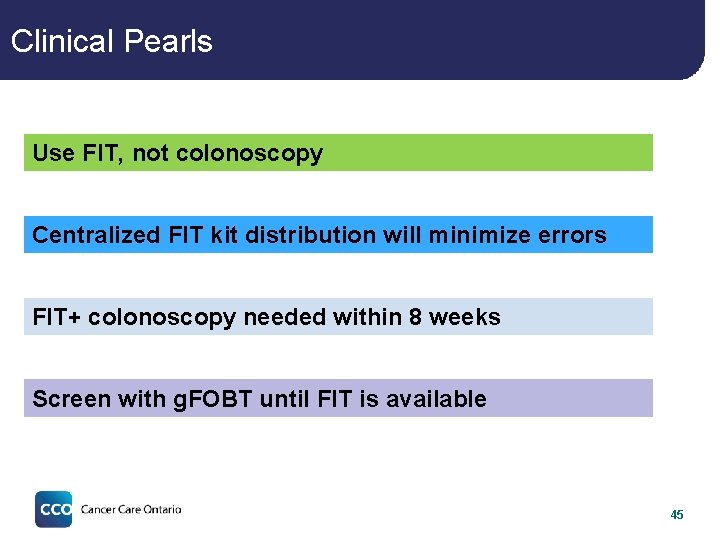
Clinical Pearls Use FIT, not colonoscopy Centralized FIT kit distribution will minimize errors FIT+ colonoscopy needed within 8 weeks Screen with g. FOBT until FIT is available 45

Appendix 46

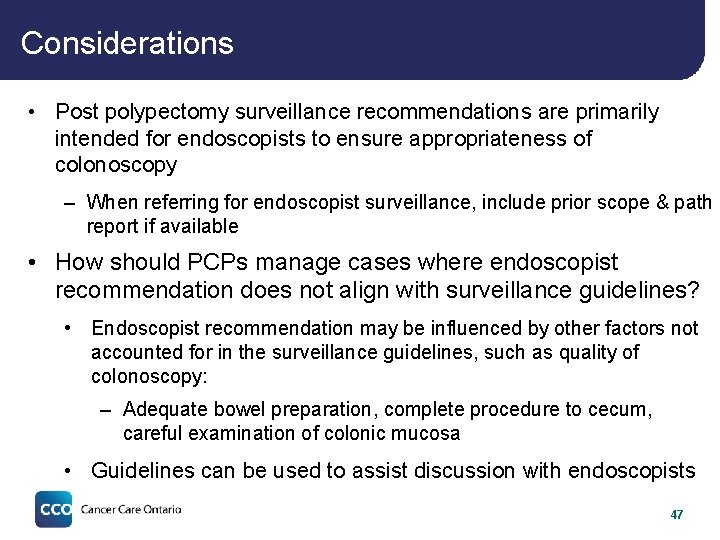
Considerations • Post polypectomy surveillance recommendations are primarily intended for endoscopists to ensure appropriateness of colonoscopy – When referring for endoscopist surveillance, include prior scope & path report if available • How should PCPs manage cases where endoscopist recommendation does not align with surveillance guidelines? • Endoscopist recommendation may be influenced by other factors not accounted for in the surveillance guidelines, such as quality of colonoscopy: – Adequate bowel preparation, complete procedure to cecum, careful examination of colonic mucosa • Guidelines can be used to assist discussion with endoscopists 47

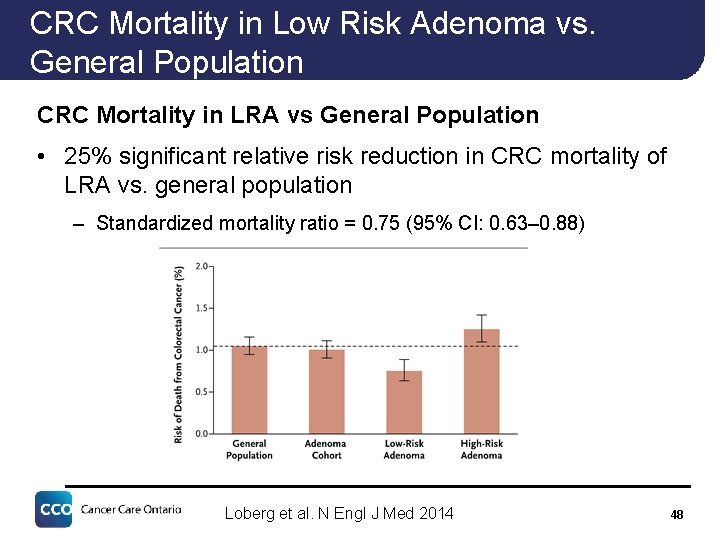
CRC Mortality in Low Risk Adenoma vs. General Population CRC Mortality in LRA vs General Population • 25% significant relative risk reduction in CRC mortality of LRA vs. general population – Standardized mortality ratio = 0. 75 (95% CI: 0. 63– 0. 88) Loberg et al. N Engl J Med 2014 48

Switching to FIT After Average Risk Colonoscopy Systematic Review: Risk of advanced neoplasia and death with low risk adenomas • No evidence to support surveillance in people with LRA – Lower risk of CRC and CRC mortality compared to the general population – Small increase in relative risk for high risk adenoma at 4 -10 years compared to those with normal colonoscopy 49

Patient Attachment • PCPs can still register to accept and roster new patients who require follow-up • Code Q 043 A or Q 053 A 50