The Evolut Low Risk Trial Complete 2 year



- Slides: 13

The Evolut Low Risk Trial Complete 2 -year Follow-up John K. Forrest, MD, Yale University School of Medicine, New Haven, CT, USA for the Evolut Low Risk Trial Investigators

Disclosures • Edwards Lifesciences: Grant Support, Advisory Board, and Proctoring. • Medtronic Inc: Grant Support, Advisory Board, and Proctoring.

Evolut Low Risk Trial: Methods • The Evolut Low Risk Trial enrolled patients with severe aortic stenosis who had a 30 -day surgical mortality risk of <3% per local heart team assessment, confirmed by a national screening committee. • The primary analysis cohort comprised patients who underwent an attempted valve implant (as-treated; 730 TAVR, 684 surgery). • A central clinical events committee adjudicated all adverse events and a central echocardiographic core laboratory assessed all echocardiograms. • When 850 patients had reached 12 -month follow-up, the primary endpoint of death or disabling stroke at 24 months was evaluated using Bayesian methods. 1 • All patients have now completed 2 -year follow up and we present here the complete 2 -year outcomes from the Evolut Low Risk Trial. 1 Popma JJ, et al. New Engl J Med; 2019; 380: 1706 -15.

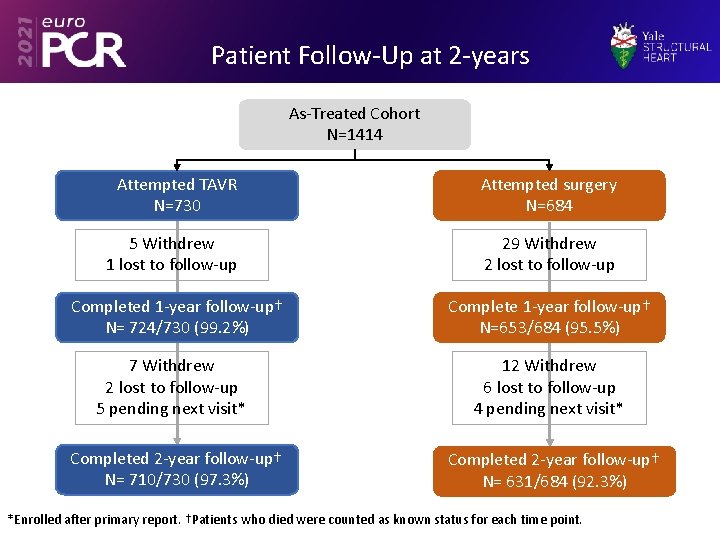
Patient Follow-Up at 2 -years As-Treated Cohort N=1414 Attempted TAVR N=730 Attempted surgery N=684 5 Withdrew 1 lost to follow-up 29 Withdrew 2 lost to follow-up Completed 1 -year follow-up† N= 724/730 (99. 2%) Complete 1 -year follow-up† N=653/684 (95. 5%) 7 Withdrew 2 lost to follow-up 5 pending next visit* 12 Withdrew 6 lost to follow-up 4 pending next visit* Completed 2 -year follow-up† N= 710/730 (97. 3%) Completed 2 -year follow-up† N= 631/684 (92. 3%) *Enrolled after primary report. †Patients who died were counted as known status for each time point.

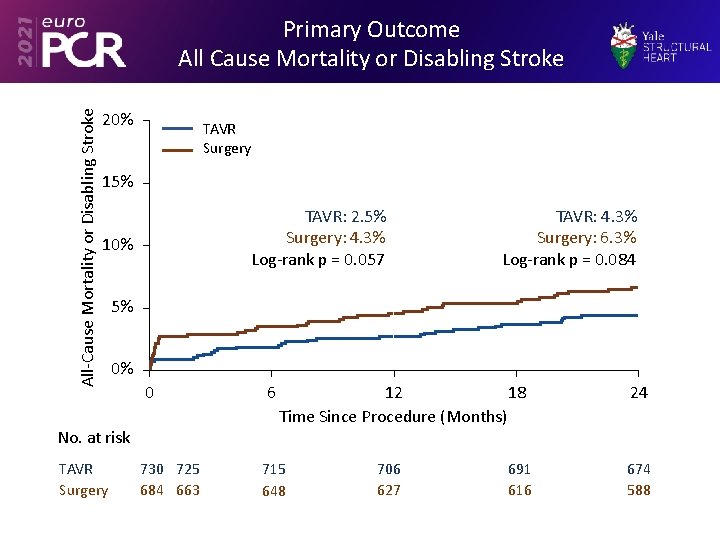
All-Cause Mortality or Disabling Stroke Primary Outcome All Cause Mortality or Disabling Stroke 20% TAVR Surgery 15% TAVR: 2. 5% Surgery: 4. 3% Log-rank p = 0. 057 10% 5% 0% 0 No. at risk TAVR Surgery TAVR: 4. 3% Surgery: 6. 3% Log-rank p = 0. 084 730 725 684 663 6 12 18 Time Since Procedure (Months) 715 648 706 627 691 616 24 674 588

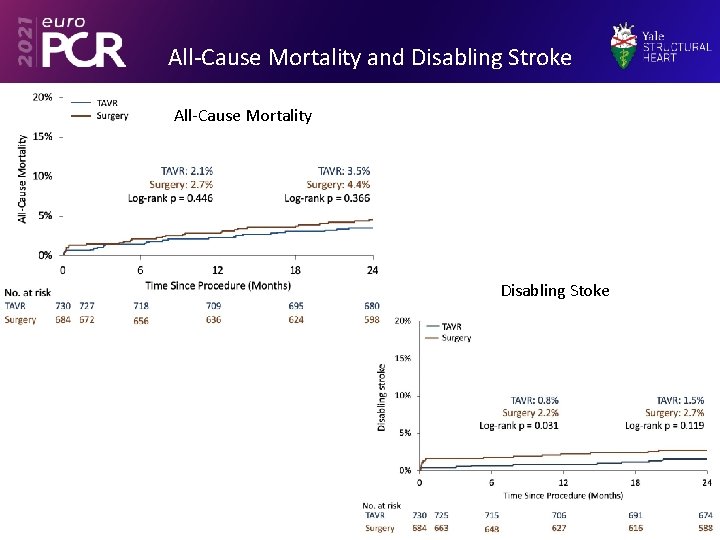
All-Cause Mortality and Disabling Stroke All-Cause Mortality Disabling Stoke

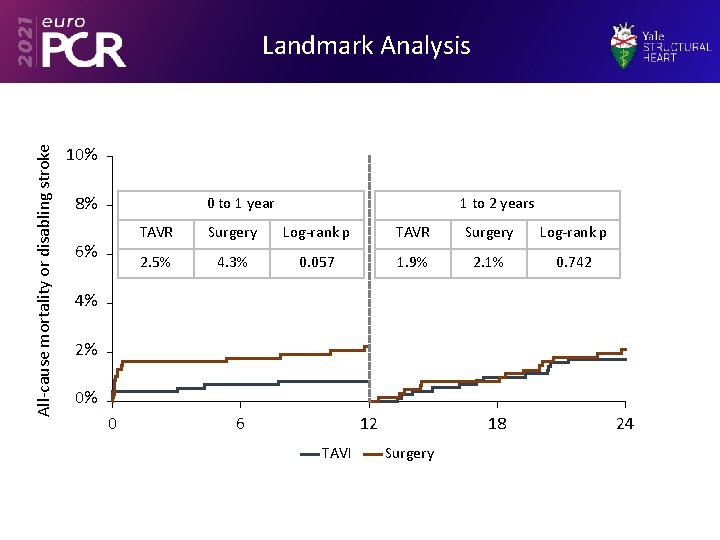
All-cause mortality or disabling stroke Landmark Analysis 10% 8% 0 to 1 year 6% 1 to 2 years TAVR Surgery Log-rank p 2. 5% 4. 3% 0. 057 1. 9% 2. 1% 0. 742 4% 2% 0% 0 6 12 TAVI 18 Surgery 24

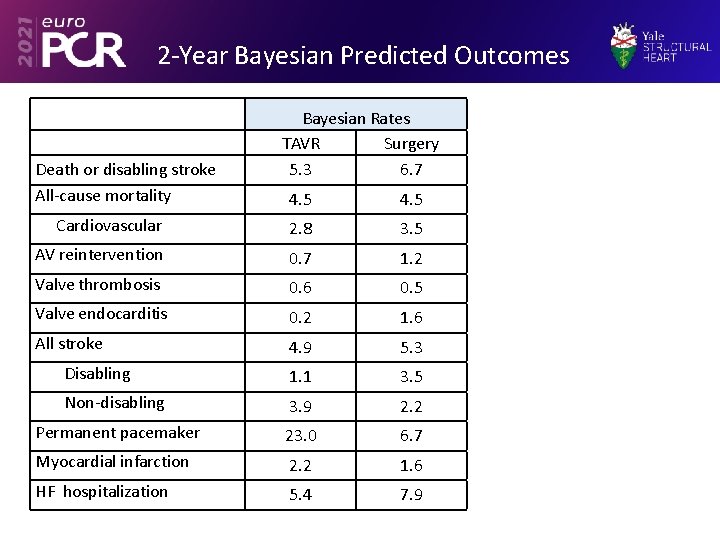
2 -Year Bayesian Predicted Outcomes Death or disabling stroke All-cause mortality Bayesian Rates TAVR Surgery 5. 3 6. 7 4. 5 Cardiovascular 2. 8 3. 5 AV reintervention 0. 7 1. 2 Valve thrombosis 0. 6 0. 5 Valve endocarditis 0. 2 1. 6 All stroke 4. 9 5. 3 Disabling 1. 1 3. 5 Non-disabling 3. 9 2. 2 Permanent pacemaker 23. 0 6. 7 Myocardial infarction 2. 2 1. 6 HF hospitalization 5. 4 7. 9

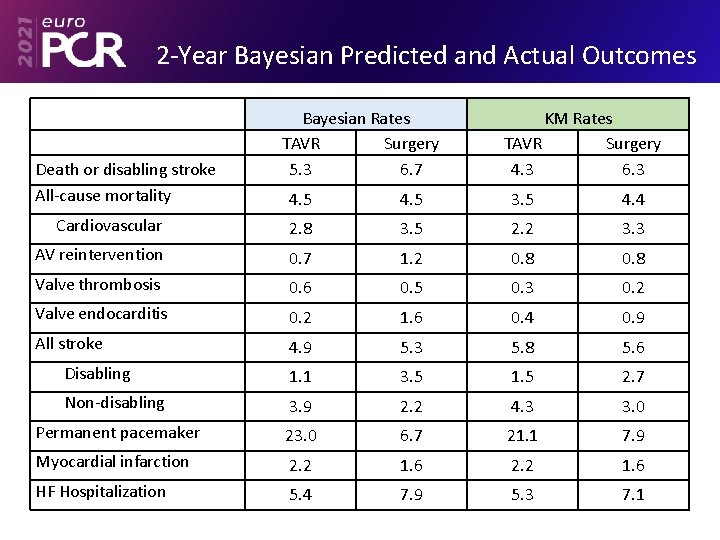
2 -Year Bayesian Predicted and Actual Outcomes Death or disabling stroke All-cause mortality Bayesian Rates TAVR Surgery 5. 3 6. 7 KM Rates TAVR Surgery 4. 3 6. 3 4. 5 3. 5 4. 4 Cardiovascular 2. 8 3. 5 2. 2 3. 3 AV reintervention 0. 7 1. 2 0. 8 Valve thrombosis 0. 6 0. 5 0. 3 0. 2 Valve endocarditis 0. 2 1. 6 0. 4 0. 9 All stroke 4. 9 5. 3 5. 8 5. 6 Disabling 1. 1 3. 5 1. 5 2. 7 Non-disabling 3. 9 2. 2 4. 3 3. 0 Permanent pacemaker 23. 0 6. 7 21. 1 7. 9 Myocardial infarction 2. 2 1. 6 HF Hospitalization 5. 4 7. 9 5. 3 7. 1

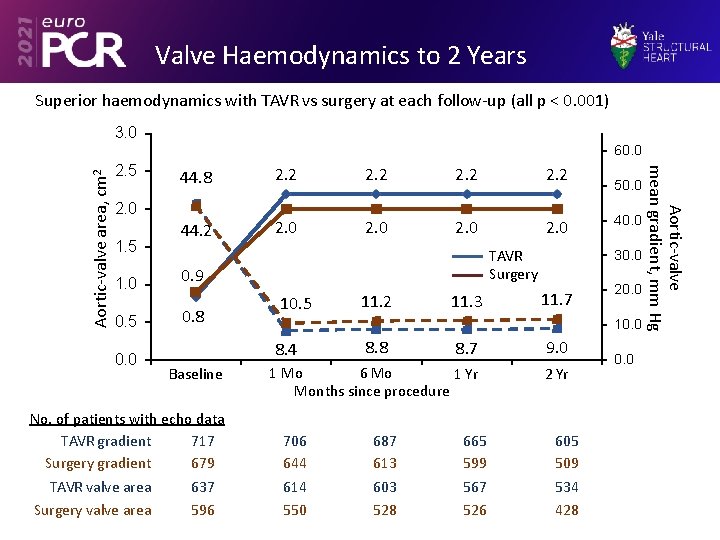
Valve Haemodynamics to 2 Years Superior haemodynamics with TAVR vs surgery at each follow-up (all p < 0. 001) 3. 0 2. 5 44. 8 2. 2 44. 2 2. 0 2. 0 1. 5 1. 0 0. 9 0. 5 0. 8 0. 0 TAVR Surgery 10. 5 No. of patients with echo data TAVR gradient 717 Surgery gradient 679 TAVR valve area 637 Surgery valve area 596 11. 3 40. 0 30. 0 11. 7 20. 0 10. 0 8. 7 9. 0 1 Mo 6 Mo 1 Yr Months since procedure 2 Yr 8. 4 Baseline 11. 2 50. 0 706 644 614 550 8. 8 687 613 603 528 665 599 567 526 605 509 534 428 0. 0 Aortic-valve mean gradient, mm Hg Aortic-valve area, cm 2 60. 0

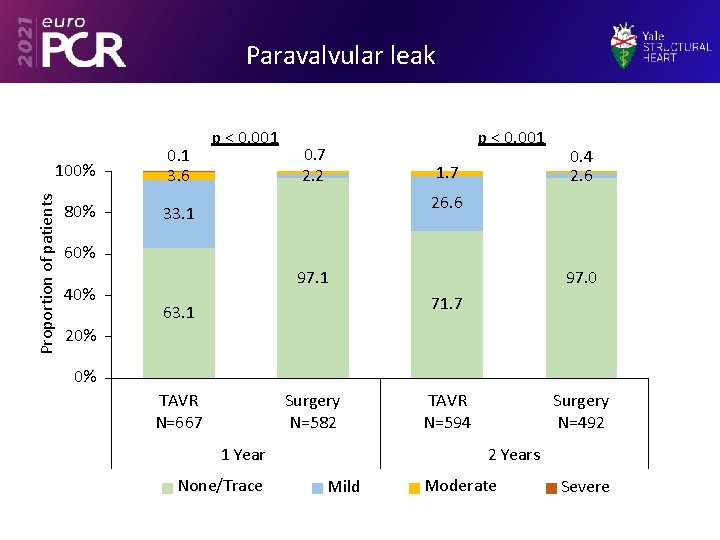
Paravalvular leak Proportion of patients 100% 80% 0. 1 3. 6 p < 0. 001 0. 7 2. 2 1. 7 0. 4 2. 6 26. 6 33. 1 60% 40% 97. 1 97. 0 71. 7 63. 1 20% 0% TAVR N=667 Surgery N=582 1 Year None/Trace TAVR N=594 Surgery N=492 2 Years Mild Moderate Severe

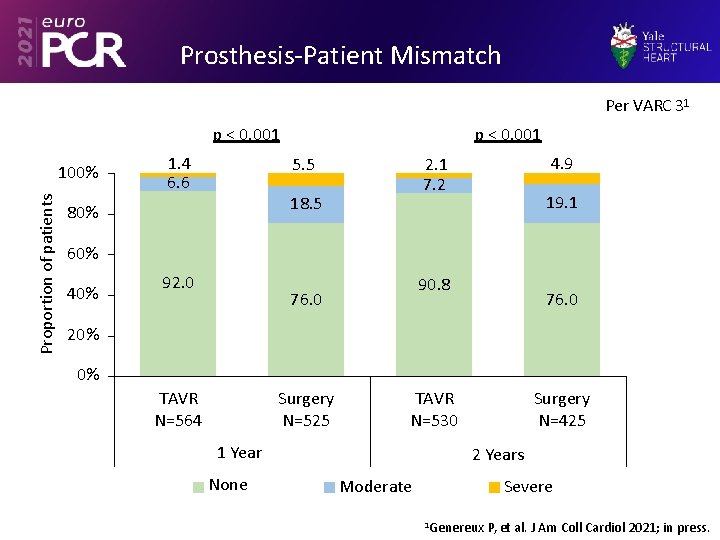
Prosthesis-Patient Mismatch Per VARC 31 p < 0. 001 Proportion of patients 100% 1. 4 6. 6 p < 0. 001 5. 5 18. 5 80% 4. 9 2. 1 7. 2 19. 1 60% 40% 92. 0 90. 8 76. 0 20% 0% TAVR N=564 Surgery N=525 TAVR N=530 1 Year None Surgery N=425 2 Years Severe Moderate 1 Genereux P, et al. J Am Coll Cardiol 2021; in press.

Summary 2 -year results from the Evolut Low Risk Trial: • The KM rate of death or disabling stroke at 2 years was 4. 3% with TAVR and 6. 3% with surgery, p = 0. 084. • Between 1 and 2 years there was no convergence of the KM curves for death or disabling stroke. • Surgery was superior to TAVR in regards to the incidence of pacemaker and ≥mild PVL. • TAVR was superior to surgery in regards to valve haemodynamics and prosthesis-patient mismatch. • Longer-term follow-up is needed to evaluate these differences and the impact on patient outcomes. Patients will be followed for 10 years.