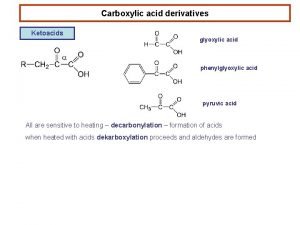
The evaluation of various oxidants used in acid



























- Slides: 28

The evaluation of various oxidants used in acid leaching of uranium SAIMM Hydrometallurgy Conference, February 2009 Riaan Venter – Senior Process Engineer


Contents • • • Introduction Mineralogy Simple chemistry Oxidants Conclusions

Introduction • In nature uranium occurs in tetravalent (U 4+) and hexavalent (U 6+) states. • U 4+ has very low solubility in acid and alkaline solutions. • U 4+ must be oxidised to U 6+ state, which has higher solubility. • Proper oxidising conditions must be maintained to achieve high uranium extraction.

Introduction When selecting an oxidant on commercial scale: • Effectiveness to maintain oxidising environment • Availability and cost – Logistics to get to site – Administering to leach

Introduction Oxidants considered • Manganese dioxide (Mn. O 2) as pyrolusite • Sodium chlorate (Na. Cl. O 3) • Hydrogen peroxide – As H 2 O 2 – As Caro’s acid (H 2 SO 5) • Oxygen in pressure leaching circuits • Sulphur dioxide/air (oxygen) mixture

Mineralogy • • • Primary uranium ores found in veins or pegmatites Secondary ores found in weathered zones of primary deposits and precipitated in sediments Multiple oxides, complex associations with rare earths

Mineralogy • Minerals containing uranium in the tetravalent state: – Uraninite (predominantly tetravalent) • Witwatersrand ores in SA • Rossing in Namibia – Coffinite • Kayelekera in Malawi • Minerals containing uranium in the hexavalent state: – Carnotite • Langer Heinrich in Namibia • Trekkopje in Namibia – Phosphate deposits • Bakouma in CAR • Commercial producers of phosphoric acid

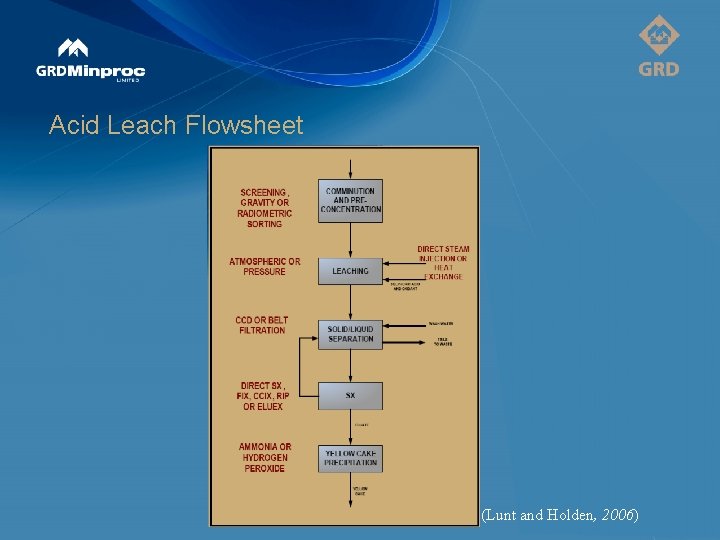
Acid Leach Flowsheet (Lunt and Holden, 2006)

Chemistry • • • U 4+ must be oxidised to U 6+ Ferric iron acts as principle oxidant Iron is present as constituent in the or introduced as metallic iron • Uranium dissolution in sulphuric acid UO 2 + 2 Fe 3+ → UO 22+ + 2 Fe 2+ + Mn. O 2 + 4 H+ → 2 Fe 3+ + Mn 2+ + 2 H 2 O

Oxidants Manganese dioxide (Mn. O 2) • Traditional oxidant used as pyrolusite on Witwatersrand ores and elsewhere • Oxidation reaction: 2 Fe 2+ + Mn. O 2 + 4 H+ → 2 Fe 3+ + Mn 2+ + 2 H 2 O • • Each mole of Mn. O 2 requires 2 moles of acid Commercially available pyrolusite contains 30% to 50% of Mn. O 2. Remainder potential acid consumers. First choice for Witwatersrand mines in the past as it was freely available. Was used in preference to SO 2/air. Vaal Reefs South Uranium Plant still operates with this flowsheet and has been for the past 30 years.

Oxidants Manganese dioxide (Mn. O 2) • Advantages: – Added as a slurry – No special agitation required and does not dissociate if not reacted immediately. • Disadvantages: – Needs milling circuit to produce slurry with fine solids – Availability and environmental considerations weighs against it. • South Africa is an exporter of manganese ores from Northern and Eastern Cape, but long distances results in increased transport costs. • Relatively low Mn. O 2 concentrations in commercially available pyrolusite results in high volumes needed to be transported to site. – Environmental effects involves Mn 2+ in solution. Pyrite oxidation will lower p. H on slimes dams and soluble manganese might enter water sources.

Oxidants Sodium Chlorate (Na. Cl. O 3) • First choice oxidant in North American uranium plants • Oxidation reaction: 6 Fe 2+ + Na. Cl. O 3 + 6 H+ → 6 Fe 3+ + Na. Cl + 3 H 2 O • Each mole of Na. Cl. O 3 requires 3 moles of acid • In South Africa traditionally not considered as a result of cheaper and more convenient alternatives • Advantages: • Na. Cl. O 3 added to the leach as a solution, i. e. no special agitation necessary • Disadvantages: • During reaction in the leach chloride ions goes into solution with adverse effects on IX and materials of construction • Sodium chlorate is relatively expensive • Auto-ignition risk when in contact with organic – needs procedures and proper engineering for safe handling

Oxidants Hydrogen Peroxide (H 2 O 2) • Used as oxidant in 1980’s in Australia, pyrolusite replaced with Caro’s Acid. • Can be used as H 2 O 2 or can be made up to Caro’s acid by reaction with H 2 SO 4 H 2 O 2 + H 2 SO 4 ↔ H 2 SO 5 + H 2 O • Oxidation reaction: Caro’s acid: 2 Fe 2+ + H 2 SO 5 + 2 H+ → 2 Fe 3+ + H 2 SO 4 + H 2 O Hydrogen peroxide: 2 Fe 2+ + H 2 O 2 + 2 H+ → 2 Fe 3+ + 2 H 2 O • Each mole of H 2 O 2 or H 2 SO 5 requires 1 mole of acid

Oxidants Hydrogen Peroxide (H 2 O 2) • Advantages: – Compared to pyrolusite: • Similar extractions to pyrolusite reported with reduction in acid consumption • Reduction in lime consumption • No manganese or gangue minerals • Cleaner oxidant handling • Simplified effluent handling • Disadvantages: – H 2 O 2 will dissociate if no rapid reaction with iron in solution – Difficulties experienced with dispersion on existing operation when changed from pyrolusite to H 2 O 2 – H 2 O 2 is hazardous to handle and transport - needs procedures and proper engineering for safe handling – Supply could become a challenge

Oxidants Hydrogen Peroxide (H 2 O 2) • Using Caro’s acid reduces H 2 O 2 consumption, more efficient use of H 2 O 2 • H 2 O 2 can also be used for precipitation of UO 4 instead of ADU, which eliminates ammonia from the flowsheet • Gold plants already using H 2 O 2 for cyanide destruction storage, use and availability is an advantage • Previously discounted based on cost, but reported to produce a purer product • Very favourable from environmental perspective, produces only water • Production of Caro’s acid was traditionally a challenge as a result of high heat generation by reaction of H 2 SO 4 with H 2 O 2. • Lately made in situ by feeding both into a funnel device with heat dissipated into slurry below.

Oxidants Oxygen • Uranium ores containing sulphidic minerals can be leached by adding oxygen at elevated temperatures and pressures • Addition of sulphides to ores that do not contain sulphidic minerals has also been proposed • Sulphuric acid and ferric sulphate are generated in-situ by reaction of oxygen with sulphides

Oxidants Oxygen • With pyrite and uraninite present the following reactions are expected: Pyrite oxidised to produce soluble iron and acid: 2 Fe. S 2 + 7 O 2 + H 2 O → 2 Fe 2 SO 4 + 2 H 2 SO 4 Ferrous iron oxidised to ferric iron by oxygen: 2 Fe. SO 4 + H 2 SO 4 + ½O 2 → Fe 2(SO 4)3 + H 2 O Iron hydrolysis removes iron at T > 170 ºC Fe 2(SO 4)3 + 6 H 2 O → 2 Fe(OH)3 + 3 H 2 SO 4 Fe 2(SO 4)3 + 3 H 2 O → Fe 2 O 3 + 3 H 2 SO 4 Jarosite also forms during hydrolysis 3 Fe 3+ + 2 SO 42 - + x. M+ + (7 -x)H 2 O → Mx(H 3 O)1 -x[Fe 3(SO 4)2(OH)6] + (5+x)H+

Oxidants Oxygen • Dissolution of uranium: U 4+ oxidised by Fe 3+ UO 2 + Fe 2(SO 4)3 → UO 2 SO 4 + 2 Fe. SO 4 Dissolution of U 4+ by oxygen UO 2 + H 2 SO 4 + ½O 2 → UO 2 SO 4 + H 2 O U 6+ dissolves in presence of H 2 SO 4 UO 3 + H 2 SO 4 → UO 2 SO 4 + H 2 O Acid consuming gangue leached by acid Ca. CO 3 + H 2 SO 4 → Ca. SO 4 + CO 2↑ + H 2 O • Reactions proceed more rapidly at high pressure and temperature

Oxidants Oxygen • Advantages: – Improved extraction – Decreased operating costs – Decreased impurities and free acid in leach solutions – Improved slurry filtration – Increased recovery of gold and uranium from pyrite • Disadvantages – Increased corrosion and maintenance – Production of soluble Si. O 2 which contaminates resin and forms crud

Oxidants Sulphur dioxide/air (SO 2/air) • Used in iron and manganese removal in cobalt circuits • First suggested by workers in USA, operating conditions and detailed chemistry determined by GML in South Africa • Oxygen in the air, together with SO 2 is responsible for oxidation 2 Fe 2+ + SO 2 + O 2 → 2 Fe 3+ + SO 42 • SO 2 and O 2 required in solution.

Oxidants Sulphur dioxide/air (SO 2/air) • Mass transfer limits maximum reaction rate as a result of lower solubility of O 2 compared to SO 2 • Oxidation rate is controlled by the SO 2/O 2 ratio and rate of oxygen mass transfer, independent of Fe 2+ concentration • Higher oxidation rates obtained in solutions than in slurries • Once SO 2 flow rate increase above O 2 mass transfer rate oxidation rate decreases as a result of reducing conditions caused by the SO 2 • Main contributor to reduced SO 2 efficiency is side reaction that produces H 2 SO 4 SO 2 + 1/2 O 2 + H 2 O → H 2 SO 4

Oxidants Sulphur dioxide/air (SO 2/air) • Large scale implementation has a number of challenges: – For slurries dispersion agitation is necessary with more installed and utilised power – SO 2 source: When taken from a sulphur burner or acid plant care must be taken to ensure that the SO 2/air ratio is satisfactory, might have higher nitrogen content – Any SO 2 escaping from the leach tanks need to be passed through a scrubbing system – Relatively low SO 2 concentrations in the gas stream gives rise to large gas flow rates – SO 2/air mixture might need to be introduced against large slurry heads which will influence the partial pressure of the gas mixture. – Oxidising Fe in a solution stream is easier but need to ensure that sufficient Fe is oxidised to oxidise the uranium

Conclusion • • • Traditionally pyrolusite was used as oxidant in acid leach of uranium in Southern Africa – Availability and logistics as well as environmental issues seem to be changing this tendency – Increased acid consumption as a result of gangue minerals SO 2/air: – Works satisfactory but has engineering challenges. – Very attractive option if acid plant on site and with low sulphur prices – Lower acid consumption, as SO 2 converted to acid Hydrogen peroxide: – An attractive alternative with no environmental effects – Reduced acid and oxidant consumption compared to pyrolusite. – Handling and transport might be a challenge and availability an issue – Might be costly compared to other oxidants

Conclusion • • Sodium chlorate: – Not really considered as a result of introduction of chlorides into the system – Also has an explosion risk Oxygen: – Works well at high temperatures and pressures. – Sulphuric acid and ferric sulphate generated in-situ

Conclusion • • • All the oxidants described work adequately in acid leaching of uranium. Possible to engineer solutions to introduce the oxidant into the leach slurry on plant scale. Factors that play a significant role in selecting a suitable oxidant: – Availability and supply of the oxidant – Cost of the oxidant – Environmental impact of the oxidant. – All three of these issues will be impacted on by the location of the plant and possible sources of oxidant close to the operation.

Acknowledgements The authors would like to thank the management of GRD Minproc (Pty)Ltd for permission to present this paper and to acknowledge the input of their colleagues in undertaking uranium projects and uranium feasibility studies.

 Positions used in nursing a patient
Positions used in nursing a patient 9-which acid is not considered a strong acid?
9-which acid is not considered a strong acid? Differentiate between acid fast and non acid fast bacteria
Differentiate between acid fast and non acid fast bacteria Hydrolysis of ether gives
Hydrolysis of ether gives Acid fast vs non acid fast
Acid fast vs non acid fast Identifying lewis acids and bases practice
Identifying lewis acids and bases practice Lewis acid bronsted acid
Lewis acid bronsted acid 7 strong acid
7 strong acid Is chloric acid a strong acid
Is chloric acid a strong acid Is sulfuric acid a weak acid
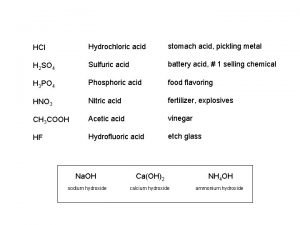
Is sulfuric acid a weak acid Stomach acid vs battery acid
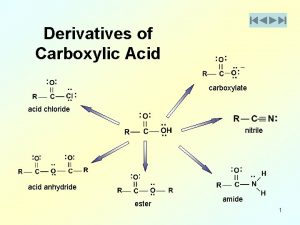
Stomach acid vs battery acid Alcoholysis of acid chlorides
Alcoholysis of acid chlorides Strong acid weak base titration graph
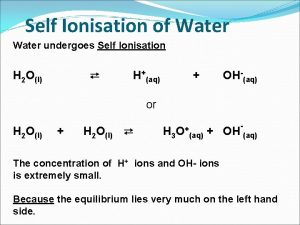
Strong acid weak base titration graph Self ionisation of water
Self ionisation of water Strong acid weak base titration
Strong acid weak base titration Monoprotic acid used in making fruit pickles
Monoprotic acid used in making fruit pickles Dilute hydrochloric acid is used in desizing
Dilute hydrochloric acid is used in desizing Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thang điểm glasgow
Thang điểm glasgow Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ