The ESSENCE Study The Efficacy and Safety of




- Slides: 50

The ESSENCE Study The Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-wave Coronary Events (Unstable angina and non-Q-wave MI) NEJM, 337: 447 -452, August, 1997

ESSENCE: Steering Committee Marc Cohen, MD Committee Chairman Robert Califf, MD Enrique Gurfinkel, MD Christine Demers, MD Anatoly Langer, MD Keith A. Fox, MB Ch. B Alexander G. Turpie, MD NEJM, 337: 447 -452, August, 1997

ESSENCE: Clinical Events Committee Marc Cohen, MD Committee Chairman Christine Demers, MD Shaun Goodman, MD Leonard Dreifus, MD John B. Kostis, MD Michael Freedman, MD Jacob Rand, MD Data and Safety Monitoring Committee Robert Makuch, Ph. D Committee Chairman John A. Cairns, MD NEJM, 337: 447 -452, August, 1997 Richard Gorlin, MD Jack Hirsh, MD

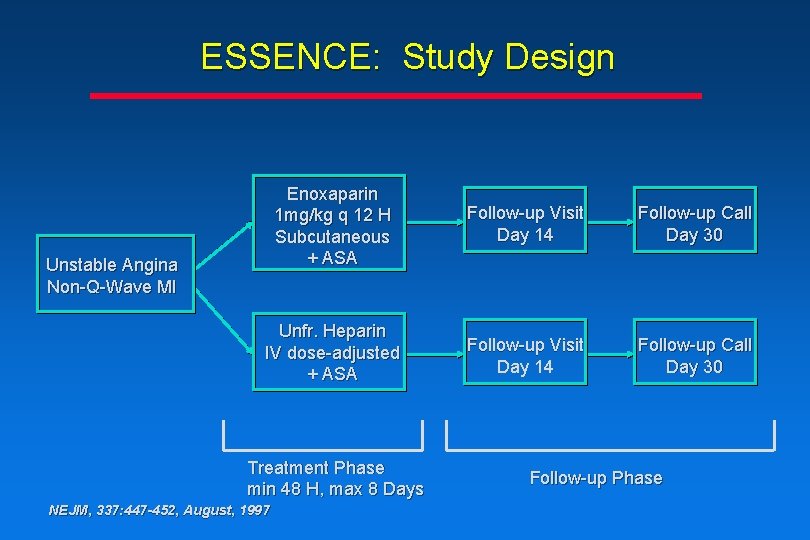
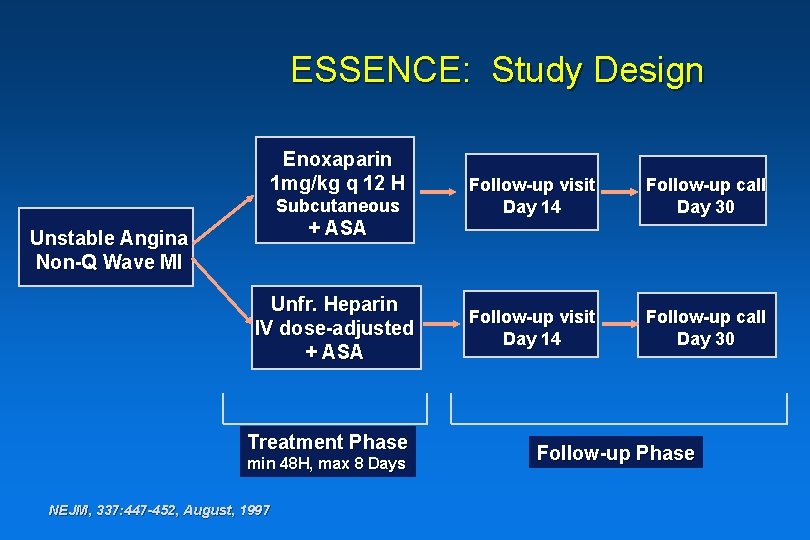
ESSENCE: Study Design Enoxaparin 1 mg/kg q 12 H Subcutaneous + ASA Follow-up Visit Day 14 Follow-up Call Day 30 Unfr. Heparin IV dose-adjusted + ASA Follow-up Visit Day 14 Follow-up Call Day 30 Unstable Angina Non-Q-Wave MI Treatment Phase min 48 H, max 8 Days NEJM, 337: 447 -452, August, 1997 Follow-up Phase

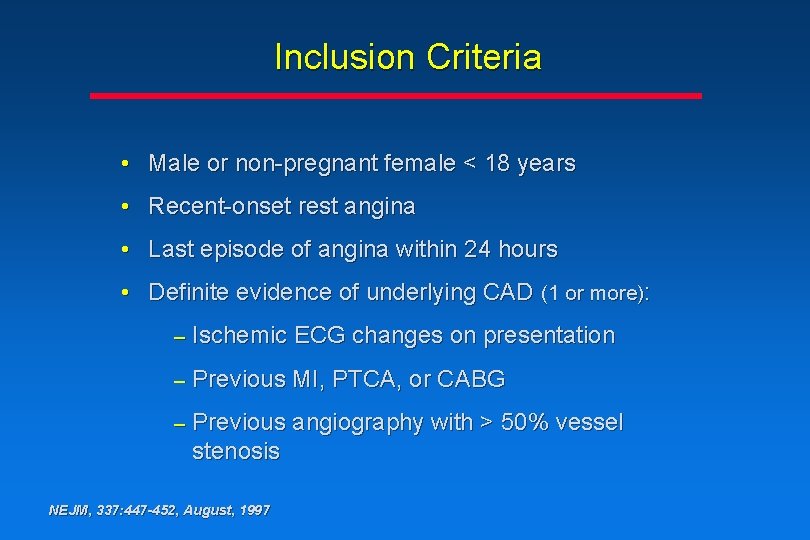
Inclusion Criteria • Male or non-pregnant female < 18 years • Recent-onset rest angina • Last episode of angina within 24 hours • Definite evidence of underlying CAD (1 or more): – Ischemic ECG changes on presentation – Previous MI, PTCA, or CABG – Previous angiography with > 50% vessel stenosis NEJM, 337: 447 -452, August, 1997

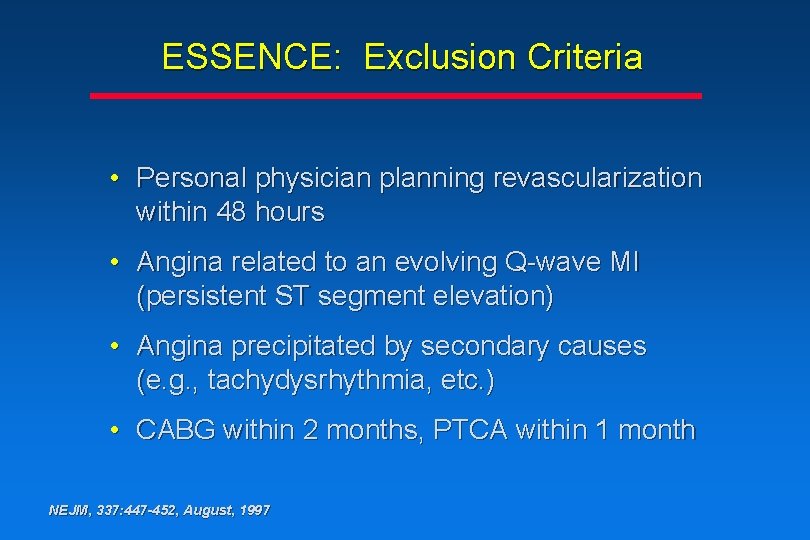
ESSENCE: Exclusion Criteria • Personal physician planning revascularization within 48 hours • Angina related to an evolving Q-wave MI (persistent ST segment elevation) • Angina precipitated by secondary causes (e. g. , tachydysrhythmia, etc. ) • CABG within 2 months, PTCA within 1 month NEJM, 337: 447 -452, August, 1997

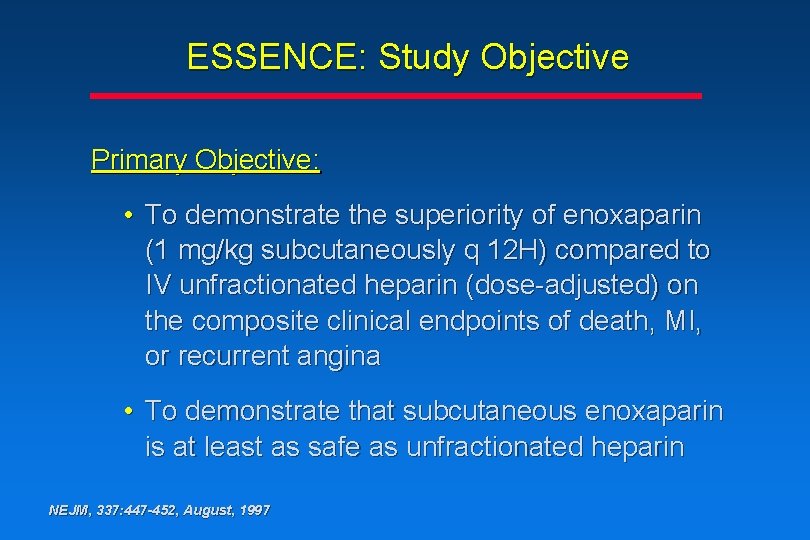
ESSENCE: Study Objective Primary Objective: • To demonstrate the superiority of enoxaparin (1 mg/kg subcutaneously q 12 H) compared to IV unfractionated heparin (dose-adjusted) on the composite clinical endpoints of death, MI, or recurrent angina • To demonstrate that subcutaneous enoxaparin is at least as safe as unfractionated heparin NEJM, 337: 447 -452, August, 1997

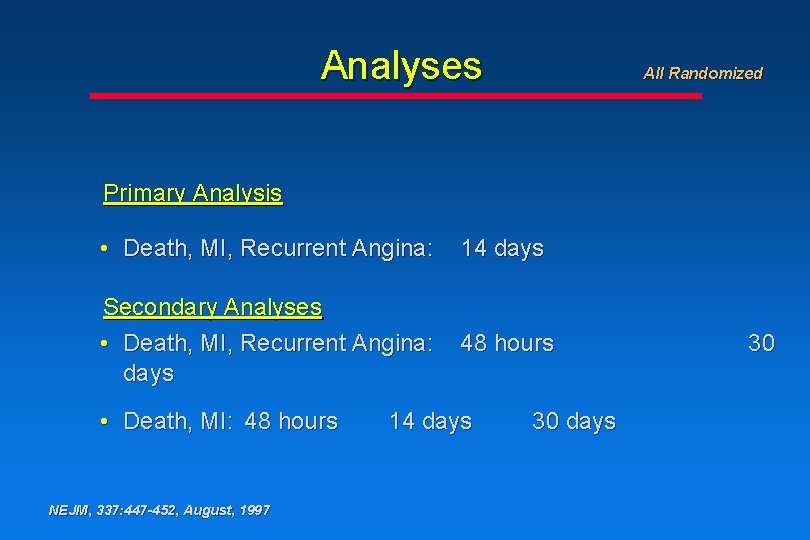
Analyses All Randomized Primary Analysis • Death, MI, Recurrent Angina: 14 days Secondary Analyses • Death, MI, Recurrent Angina: days 48 hours • Death, MI: 48 hours NEJM, 337: 447 -452, August, 1997 14 days 30

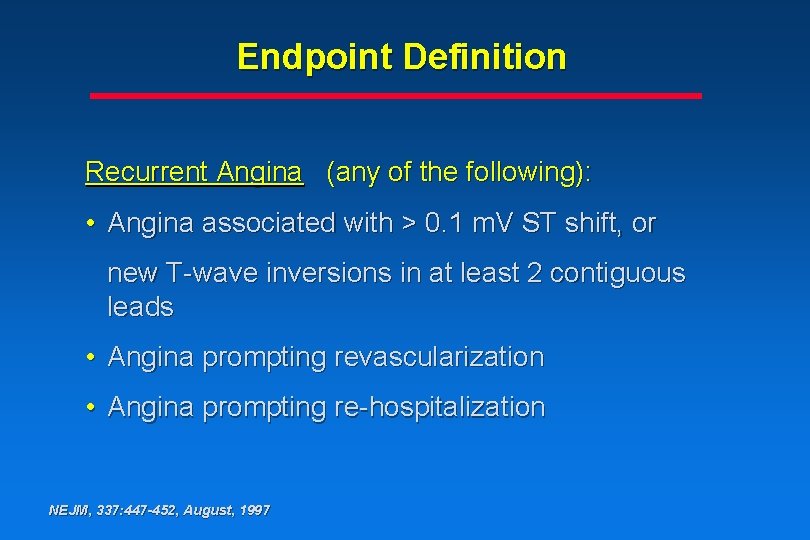
Endpoint Definition Recurrent Angina (any of the following): • Angina associated with > 0. 1 m. V ST shift, or new T-wave inversions in at least 2 contiguous leads • Angina prompting revascularization • Angina prompting re-hospitalization NEJM, 337: 447 -452, August, 1997

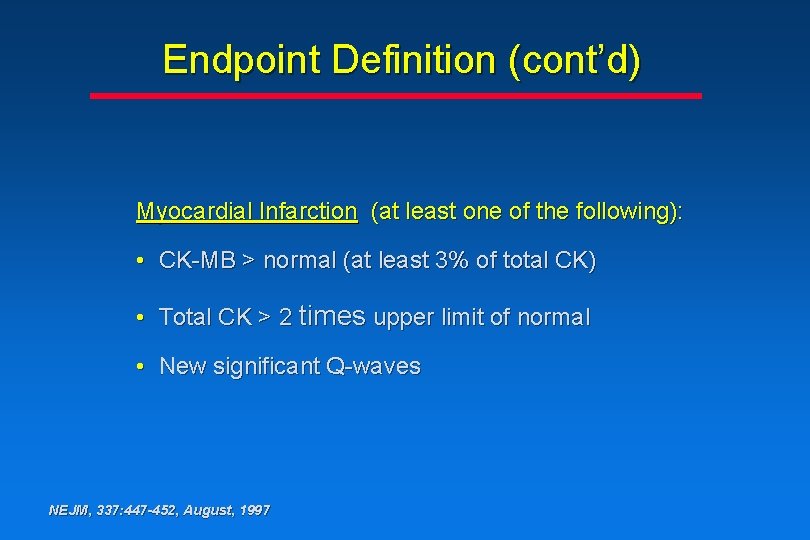
Endpoint Definition (cont’d) Myocardial Infarction (at least one of the following): • CK-MB > normal (at least 3% of total CK) • Total CK > 2 times upper limit of normal • New significant Q-waves NEJM, 337: 447 -452, August, 1997

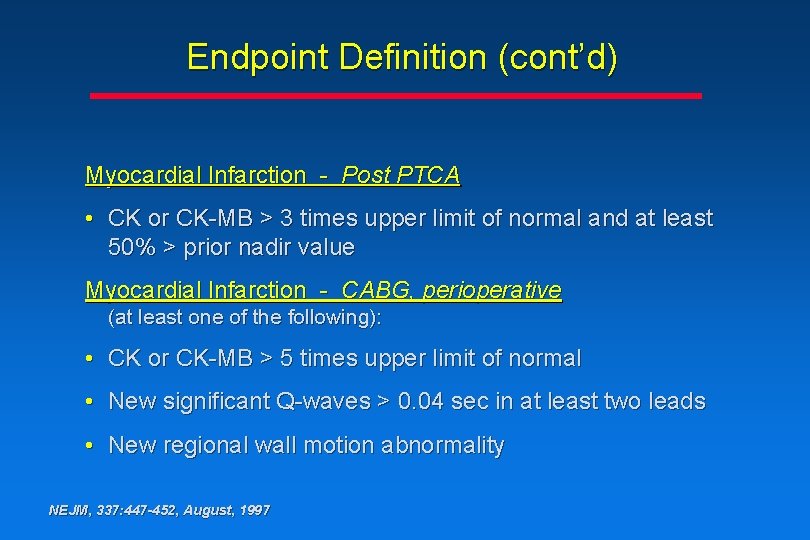
Endpoint Definition (cont’d) Myocardial Infarction - Post PTCA • CK or CK-MB > 3 times upper limit of normal and at least 50% > prior nadir value Myocardial Infarction - CABG, perioperative (at least one of the following): • CK or CK-MB > 5 times upper limit of normal • New significant Q-waves > 0. 04 sec in at least two leads • New regional wall motion abnormality NEJM, 337: 447 -452, August, 1997

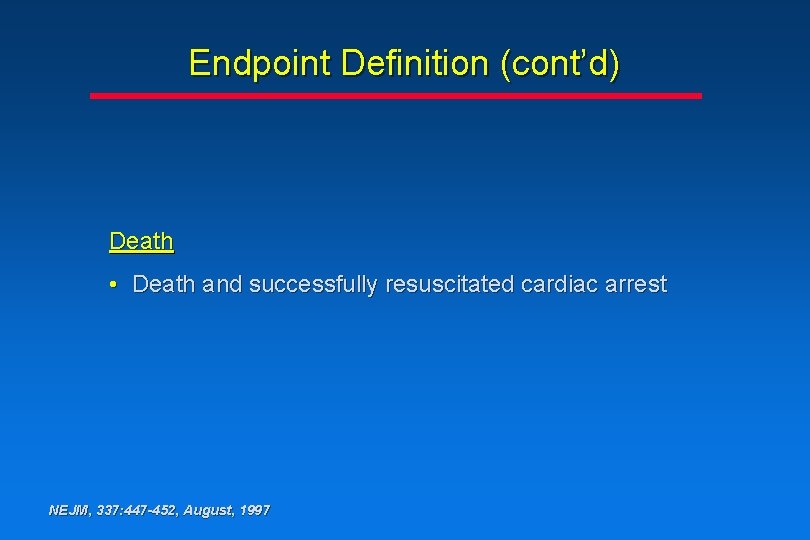
Endpoint Definition (cont’d) Death • Death and successfully resuscitated cardiac arrest NEJM, 337: 447 -452, August, 1997

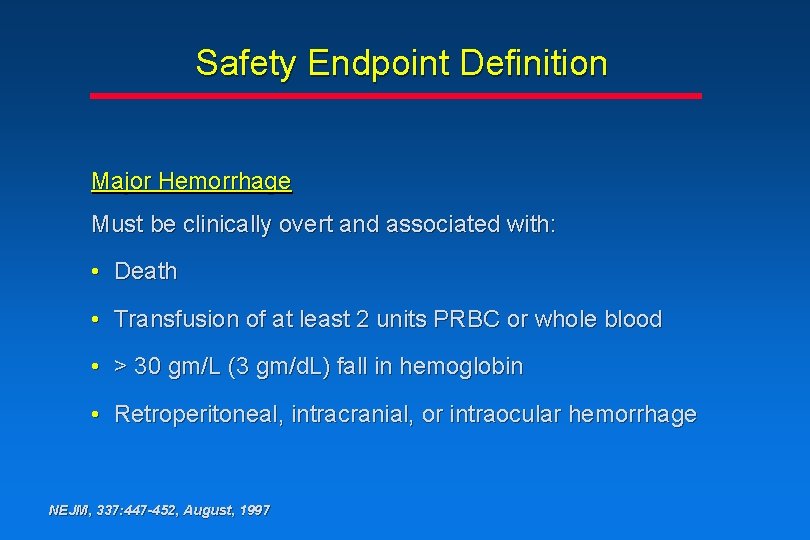
Safety Endpoint Definition Major Hemorrhage Must be clinically overt and associated with: • Death • Transfusion of at least 2 units PRBC or whole blood • > 30 gm/L (3 gm/d. L) fall in hemoglobin • Retroperitoneal, intracranial, or intraocular hemorrhage NEJM, 337: 447 -452, August, 1997

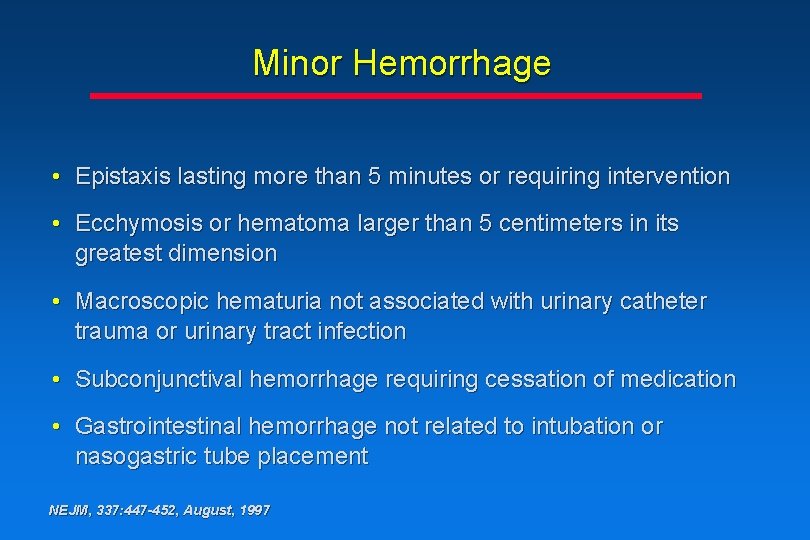
Minor Hemorrhage • Epistaxis lasting more than 5 minutes or requiring intervention • Ecchymosis or hematoma larger than 5 centimeters in its greatest dimension • Macroscopic hematuria not associated with urinary catheter trauma or urinary tract infection • Subconjunctival hemorrhage requiring cessation of medication • Gastrointestinal hemorrhage not related to intubation or nasogastric tube placement NEJM, 337: 447 -452, August, 1997

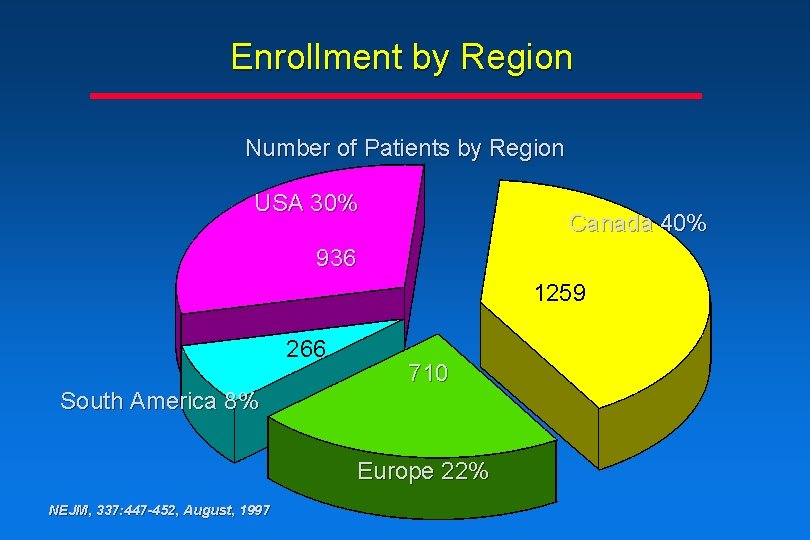
Enrollment by Region Number of Patients by Region USA 30% Canada 40% 936 1259 266 710 South America 8% Europe 22% NEJM, 337: 447 -452, August, 1997

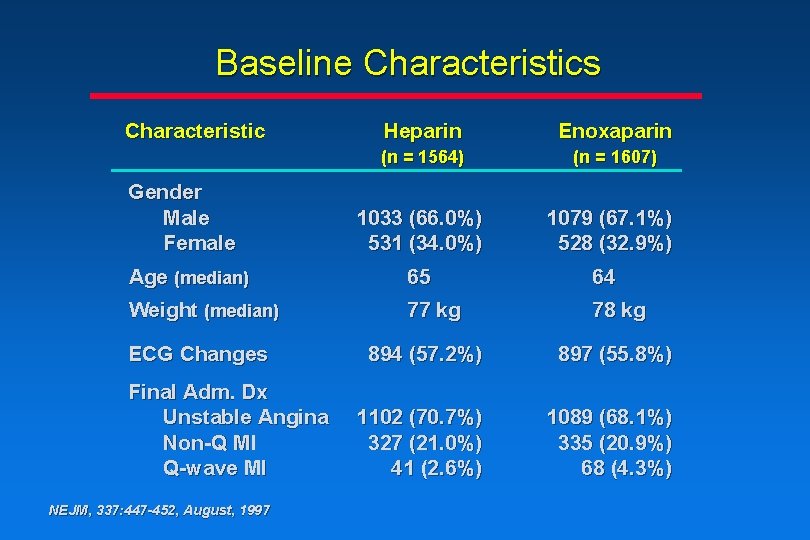
Baseline Characteristics Characteristic Gender Male Female Heparin Enoxaparin (n = 1564) (n = 1607) 1033 (66. 0%) 531 (34. 0%) 1079 (67. 1%) 528 (32. 9%) Age (median) 65 64 Weight (median) 77 kg 78 kg ECG Changes Final Adm. Dx Unstable Angina Non-Q MI Q-wave MI NEJM, 337: 447 -452, August, 1997 894 (57. 2%) 897 (55. 8%) 1102 (70. 7%) 327 (21. 0%) 41 (2. 6%) 1089 (68. 1%) 335 (20. 9%) 68 (4. 3%)

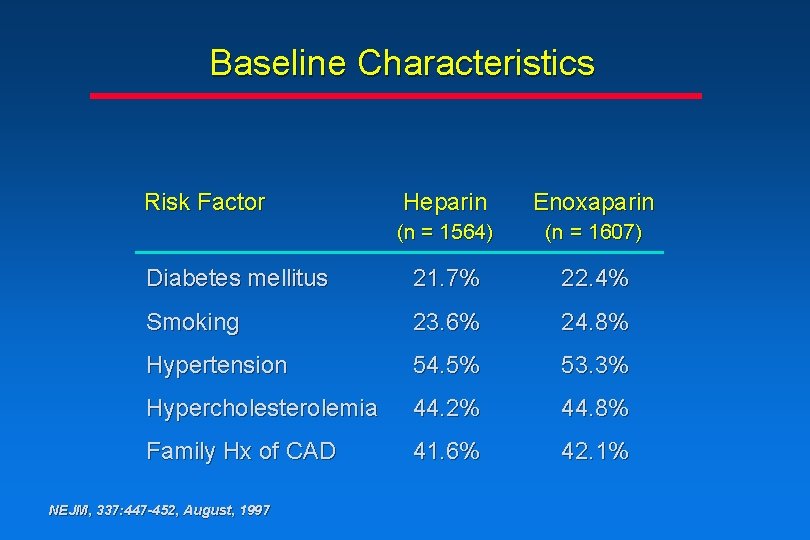
Baseline Characteristics Risk Factor Heparin Enoxaparin (n = 1564) (n = 1607) Diabetes mellitus 21. 7% 22. 4% Smoking 23. 6% 24. 8% Hypertension 54. 5% 53. 3% Hypercholesterolemia 44. 2% 44. 8% Family Hx of CAD 41. 6% 42. 1% NEJM, 337: 447 -452, August, 1997

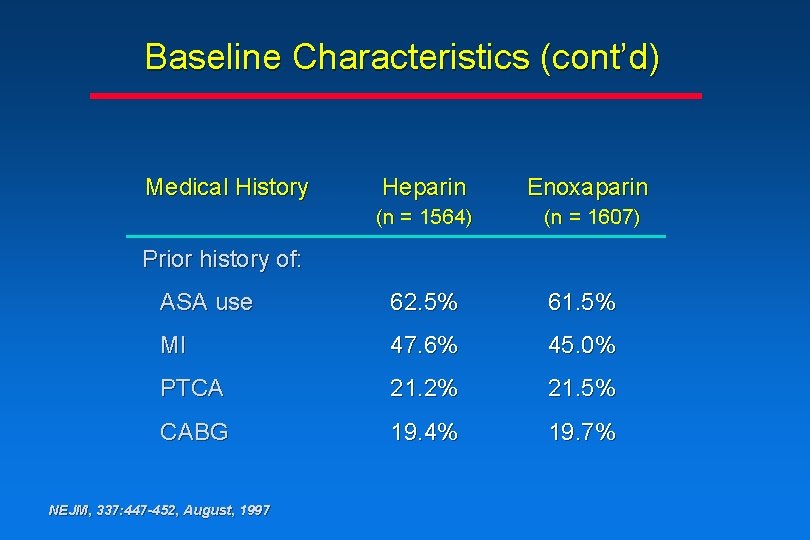
Baseline Characteristics (cont’d) Medical History Heparin Enoxaparin (n = 1564) (n = 1607) Prior history of: ASA use 62. 5% 61. 5% MI 47. 6% 45. 0% PTCA 21. 2% 21. 5% CABG 19. 4% 19. 7% NEJM, 337: 447 -452, August, 1997

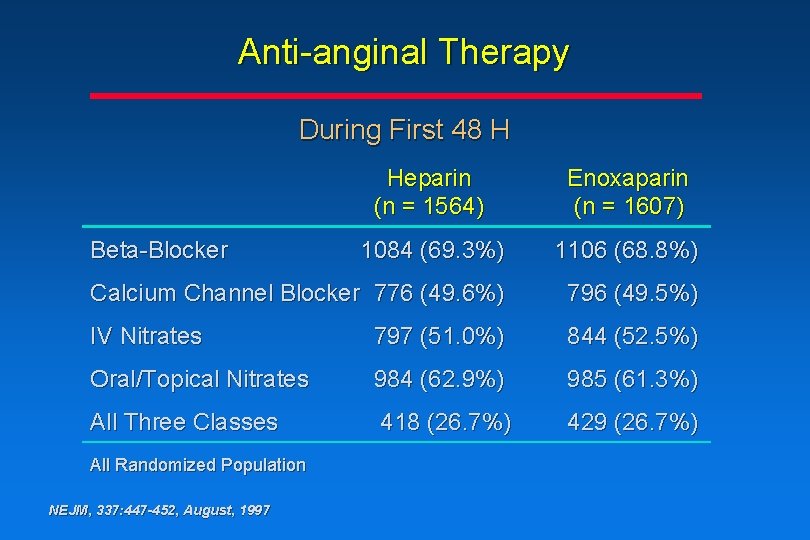
Anti-anginal Therapy During First 48 H Heparin (n = 1564) Enoxaparin (n = 1607) 1084 (69. 3%) 1106 (68. 8%) Calcium Channel Blocker 776 (49. 6%) 796 (49. 5%) IV Nitrates 797 (51. 0%) 844 (52. 5%) Oral/Topical Nitrates 984 (62. 9%) 985 (61. 3%) All Three Classes 418 (26. 7%) 429 (26. 7%) Beta-Blocker All Randomized Population NEJM, 337: 447 -452, August, 1997

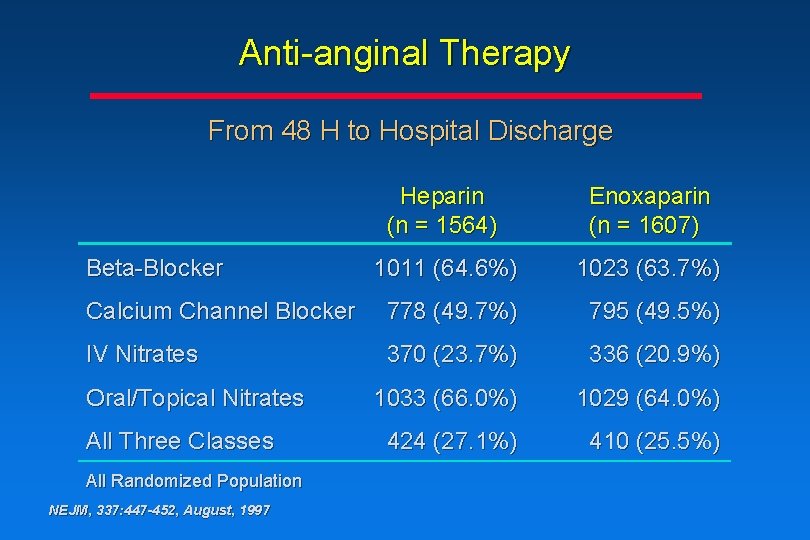
Anti-anginal Therapy From 48 H to Hospital Discharge Heparin (n = 1564) Enoxaparin (n = 1607) 1011 (64. 6%) 1023 (63. 7%) Calcium Channel Blocker 778 (49. 7%) 795 (49. 5%) IV Nitrates 370 (23. 7%) 336 (20. 9%) 1033 (66. 0%) 1029 (64. 0%) 424 (27. 1%) 410 (25. 5%) Beta-Blocker Oral/Topical Nitrates All Three Classes All Randomized Population NEJM, 337: 447 -452, August, 1997

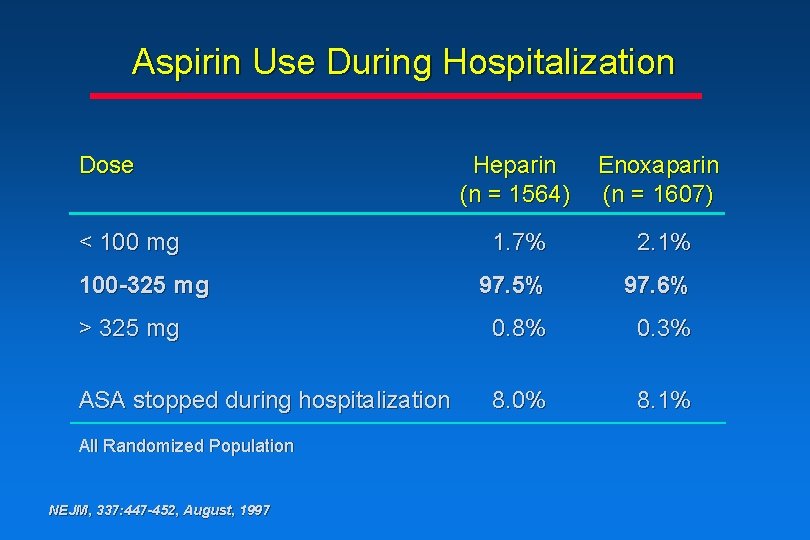
Aspirin Use During Hospitalization Dose Heparin (n = 1564) Enoxaparin (n = 1607) 1. 7% 2. 1% 97. 5% 97. 6% > 325 mg 0. 8% 0. 3% ASA stopped during hospitalization 8. 0% 8. 1% < 100 mg 100 -325 mg All Randomized Population NEJM, 337: 447 -452, August, 1997

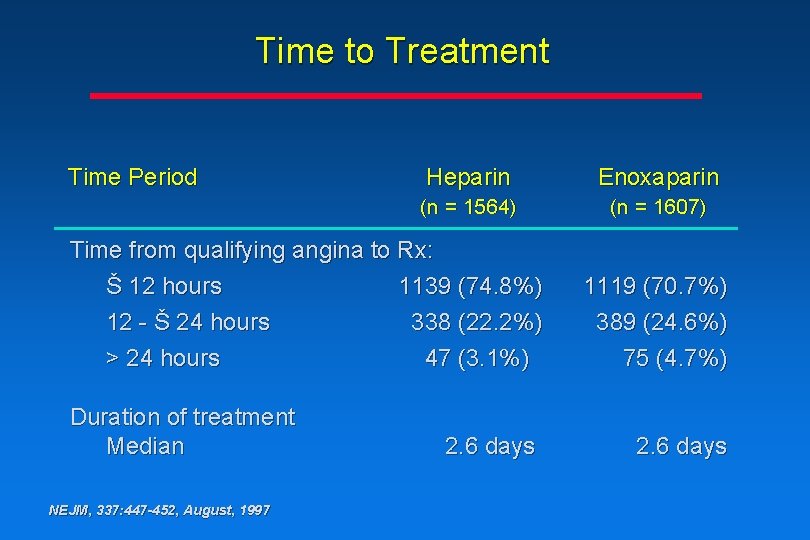
Time to Treatment Time Period Heparin Enoxaparin (n = 1564) (n = 1607) Time from qualifying angina to Rx: Š 12 hours 1139 (74. 8%) 12 - Š 24 hours 338 (22. 2%) > 24 hours 47 (3. 1%) Duration of treatment Median NEJM, 337: 447 -452, August, 1997 2. 6 days 1119 (70. 7%) 389 (24. 6%) 75 (4. 7%) 2. 6 days

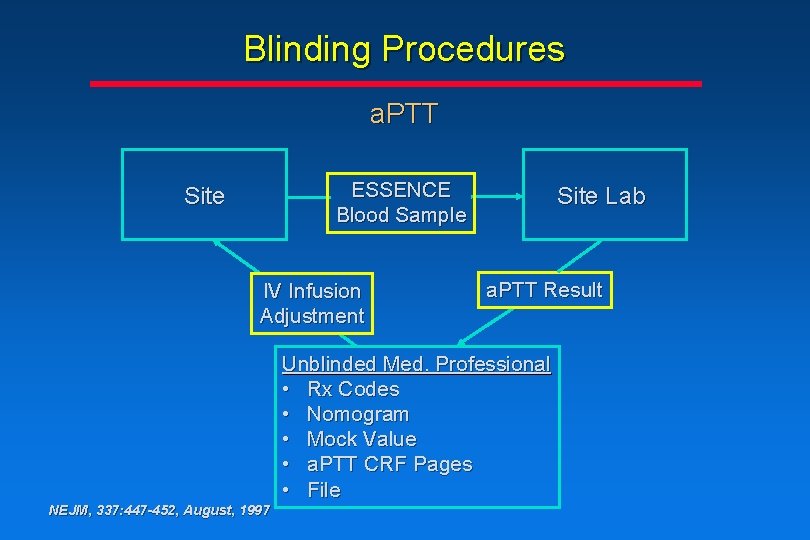
Blinding Procedures a. PTT ESSENCE Blood Sample Site IV Infusion Adjustment Site Lab a. PTT Result Unblinded Med. Professional • Rx Codes • Nomogram • Mock Value • a. PTT CRF Pages • File NEJM, 337: 447 -452, August, 1997

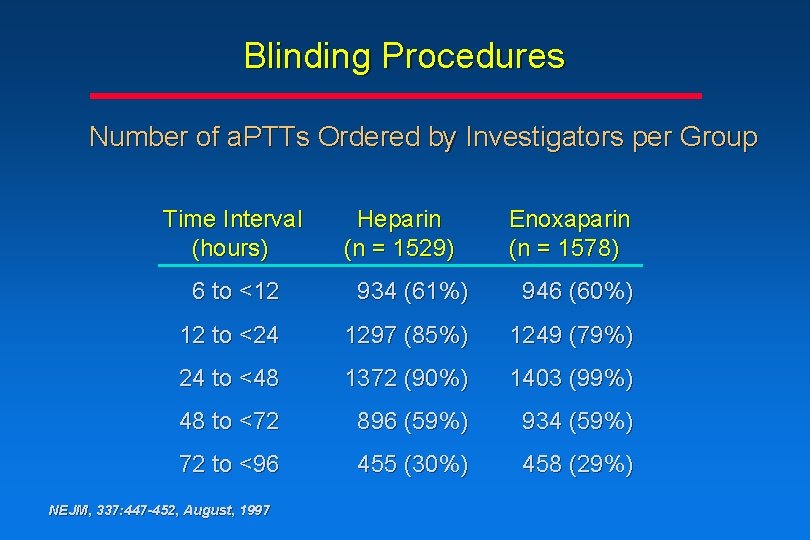
Blinding Procedures Number of a. PTTs Ordered by Investigators per Group Time Interval (hours) Heparin (n = 1529) Enoxaparin (n = 1578) 6 to <12 934 (61%) 946 (60%) 12 to <24 1297 (85%) 1249 (79%) 24 to <48 1372 (90%) 1403 (99%) 48 to <72 896 (59%) 934 (59%) 72 to <96 455 (30%) 458 (29%) NEJM, 337: 447 -452, August, 1997

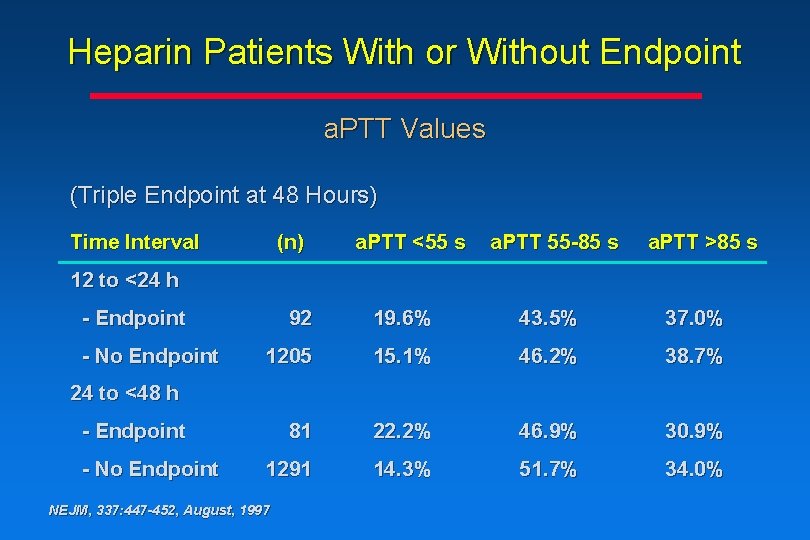
Heparin Patients With or Without Endpoint a. PTT Values (Triple Endpoint at 48 Hours) Time Interval (n) a. PTT <55 s a. PTT 55 -85 s a. PTT >85 s 12 to <24 h - Endpoint - No Endpoint 92 19. 6% 43. 5% 37. 0% 1205 15. 1% 46. 2% 38. 7% 81 22. 2% 46. 9% 30. 9% 1291 14. 3% 51. 7% 34. 0% 24 to <48 h - Endpoint - No Endpoint NEJM, 337: 447 -452, August, 1997

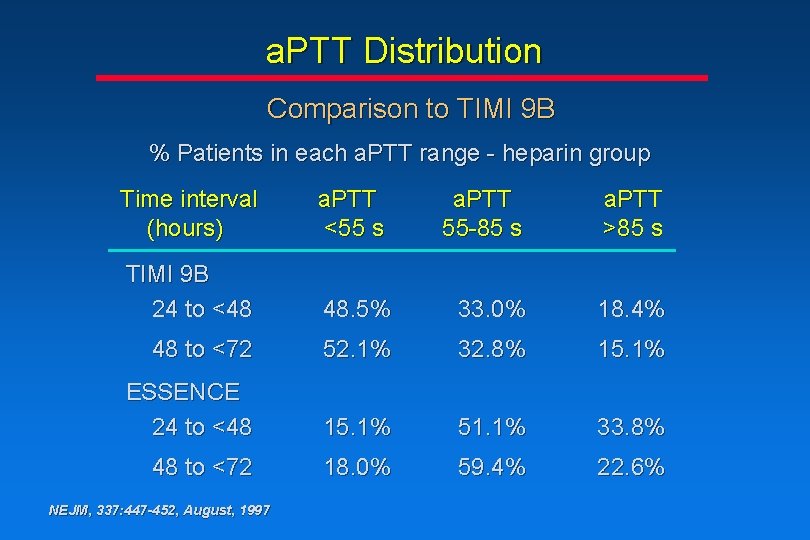
a. PTT Distribution Comparison to TIMI 9 B % Patients in each a. PTT range - heparin group Time interval (hours) a. PTT <55 s a. PTT 55 -85 s a. PTT >85 s TIMI 9 B 24 to <48 48. 5% 33. 0% 18. 4% 48 to <72 52. 1% 32. 8% 15. 1% ESSENCE 24 to <48 15. 1% 51. 1% 33. 8% 48 to <72 18. 0% 59. 4% 22. 6% NEJM, 337: 447 -452, August, 1997

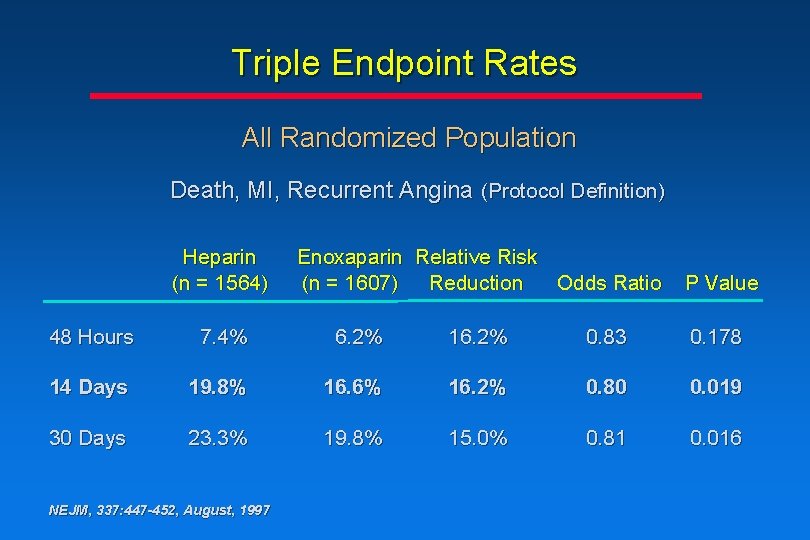
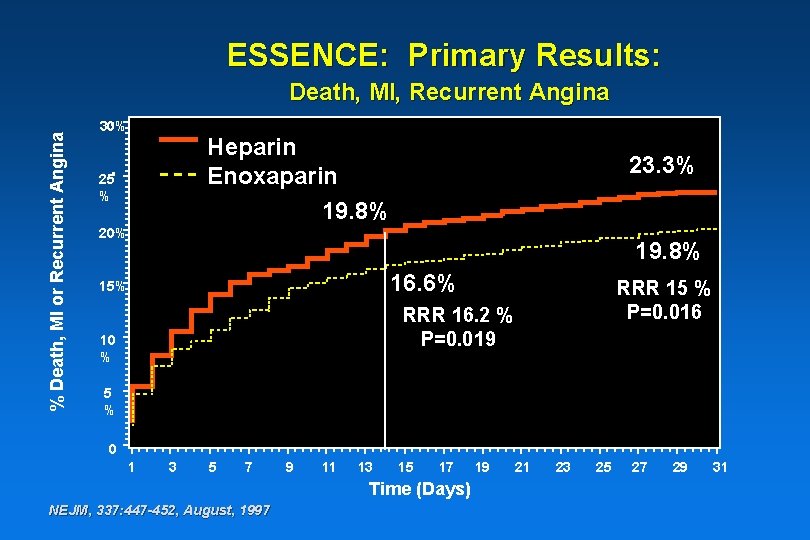
Triple Endpoint Rates All Randomized Population Death, MI, Recurrent Angina (Protocol Definition) Heparin (n = 1564) Enoxaparin Relative Risk (n = 1607) Reduction Odds Ratio P Value 48 Hours 7. 4% 6. 2% 16. 2% 0. 83 0. 178 14 Days 19. 8% 16. 6% 16. 2% 0. 80 0. 019 30 Days 23. 3% 19. 8% 15. 0% 0. 81 0. 016 NEJM, 337: 447 -452, August, 1997

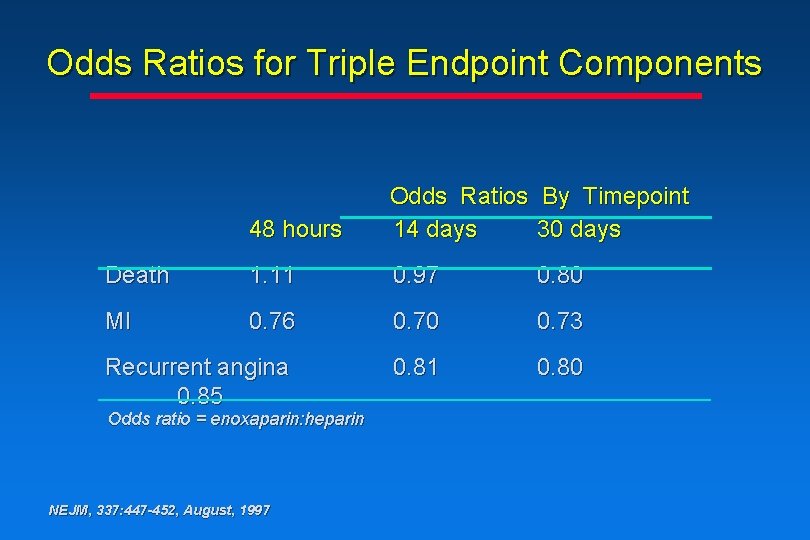
Odds Ratios for Triple Endpoint Components 48 hours Odds Ratios By Timepoint 14 days 30 days Death 1. 11 0. 97 0. 80 MI 0. 76 0. 70 0. 73 Recurrent angina 0. 85 0. 81 0. 80 Odds ratio = enoxaparin: heparin NEJM, 337: 447 -452, August, 1997

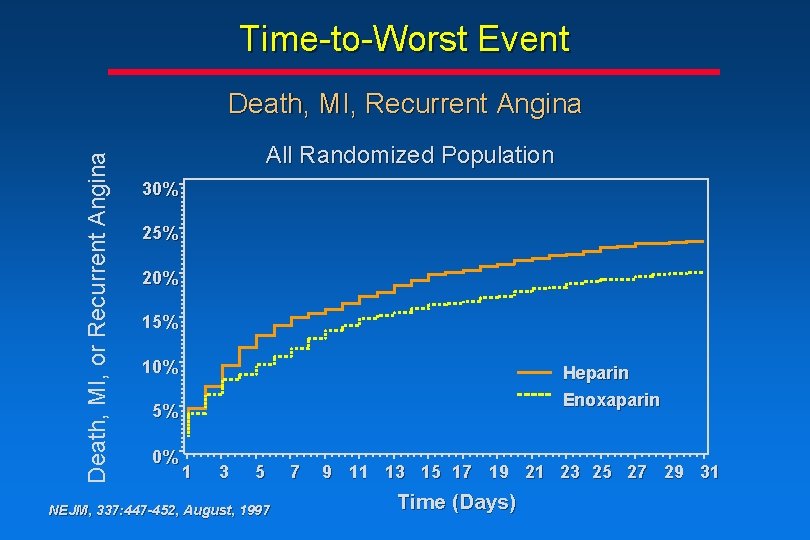
Time-to-Worst Event Death, MI, or Recurrent Angina Death, MI, Recurrent Angina All Randomized Population 30% 25% 20% 15% 10% Heparin Enoxaparin 5% 0% 1 3 5 NEJM, 337: 447 -452, August, 1997 7 9 11 13 15 17 19 21 23 25 27 29 31 Time (Days)

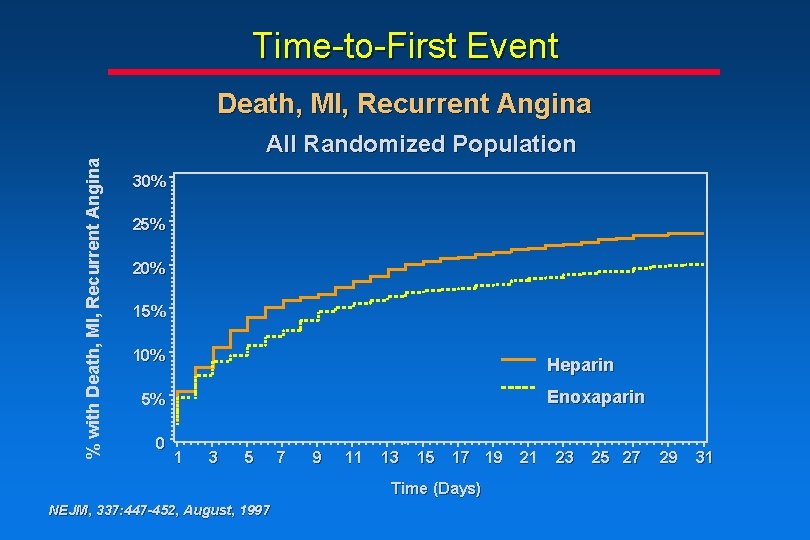
Time-to-First Event % with Death, MI, Recurrent Angina All Randomized Population 30% 25% 20% 15% 10% Heparin Enoxaparin 5% 0 1 3 5 7 9 11 13 15 17 19 Time (Days) NEJM, 337: 447 -452, August, 1997 21 23 25 27 29 31

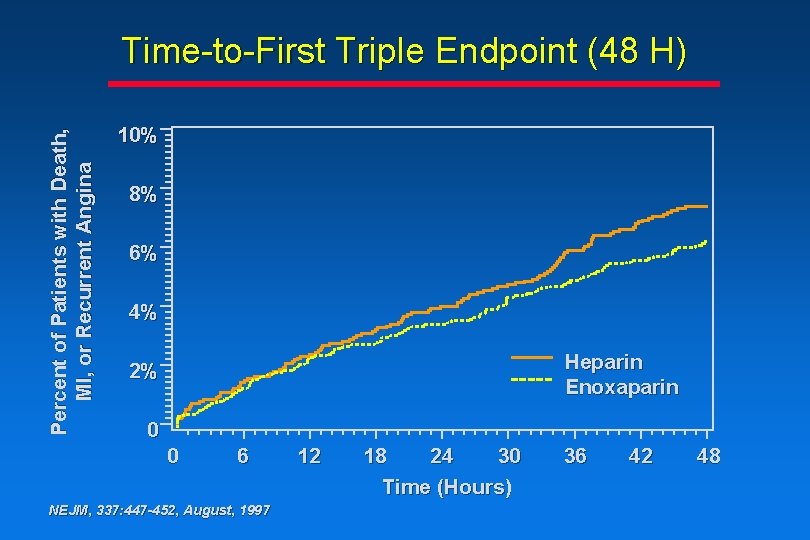
Percent of Patients with Death, MI, or Recurrent Angina Time-to-First Triple Endpoint (48 H) 10% 8% 6% 4% Heparin Enoxaparin 2% 0 0 6 NEJM, 337: 447 -452, August, 1997 12 18 24 30 Time (Hours) 36 42 48

ESSENCE: Study Design Enoxaparin 1 mg/kg q 12 H Subcutaneous + ASA Unstable Angina Non-Q Wave MI Unfr. Heparin IV dose-adjusted + ASA Treatment Phase min 48 H, max 8 Days NEJM, 337: 447 -452, August, 1997 Follow-up visit Day 14 Follow-up call Day 30 Follow-up Phase

ESSENCE: Primary Results: % Death, MI or Recurrent Angina Death, MI, Recurrent Angina 30% Heparin Enoxaparin 19. 8% 25 % 23. 3% 20% 19. 8% 16. 6% 15% RRR 15 % P=0. 016 RRR 16. 2 % P=0. 019 10 % 5 % 0 1 3 5 7 9 11 13 15 17 Time (Days) NEJM, 337: 447 -452, August, 1997 19 21 23 25 27 29 31

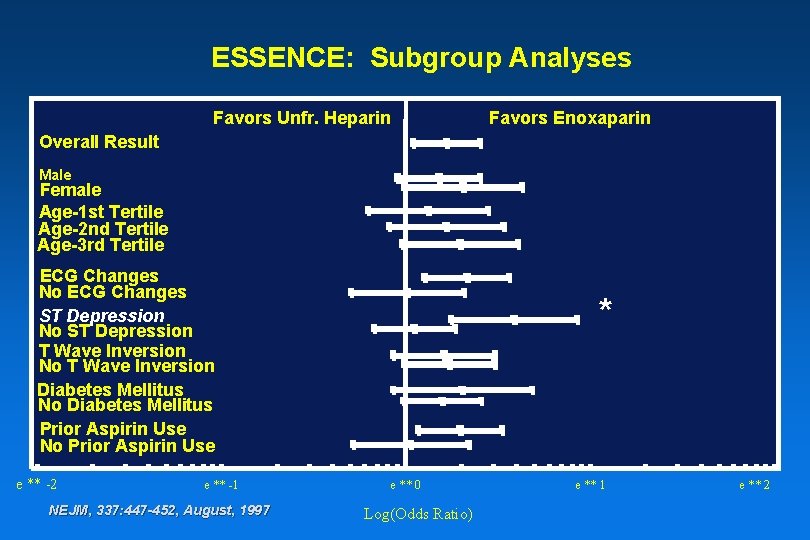
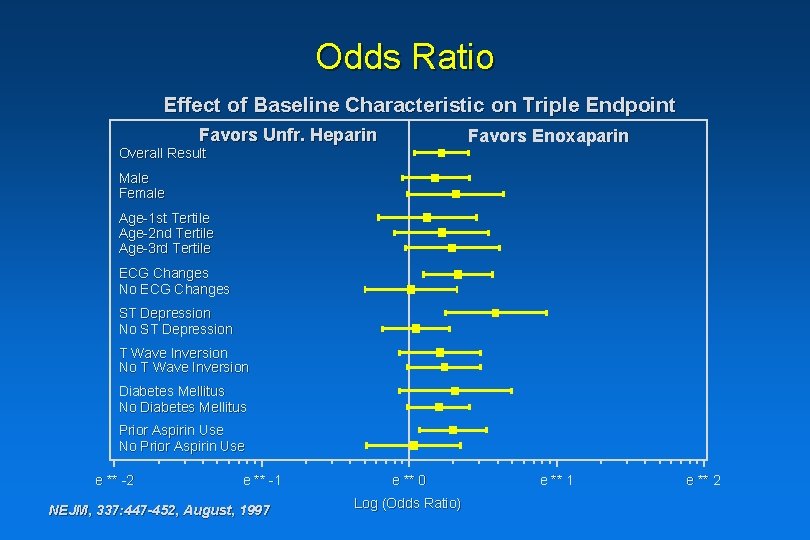
ESSENCE: Subgroup Analyses Favors Unfr. Heparin Favors Enoxaparin Overall Result Male Female Age-1 st Tertile Age-2 nd Tertile Age-3 rd Tertile ECG Changes No ECG Changes ST Depression No ST Depression T Wave Inversion No T Wave Inversion Diabetes Mellitus No Diabetes Mellitus Prior Aspirin Use No Prior Aspirin Use e ** -2 e ** -1 NEJM, 337: 447 -452, August, 1997 * e ** 0 Log(Odds Ratio) e ** 1 e ** 2

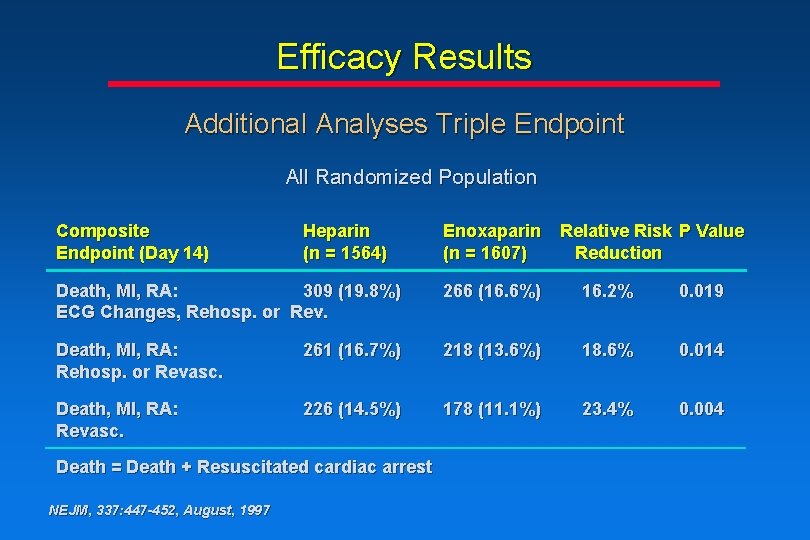
Efficacy Results Additional Analyses Triple Endpoint All Randomized Population Composite Endpoint (Day 14) Heparin (n = 1564) Enoxaparin Relative Risk P Value (n = 1607) Reduction Death, MI, RA: 309 (19. 8%) ECG Changes, Rehosp. or Rev. 266 (16. 6%) 16. 2% 0. 019 Death, MI, RA: Rehosp. or Revasc. 261 (16. 7%) 218 (13. 6%) 18. 6% 0. 014 Death, MI, RA: Revasc. 226 (14. 5%) 178 (11. 1%) 23. 4% 0. 004 Death = Death + Resuscitated cardiac arrest NEJM, 337: 447 -452, August, 1997

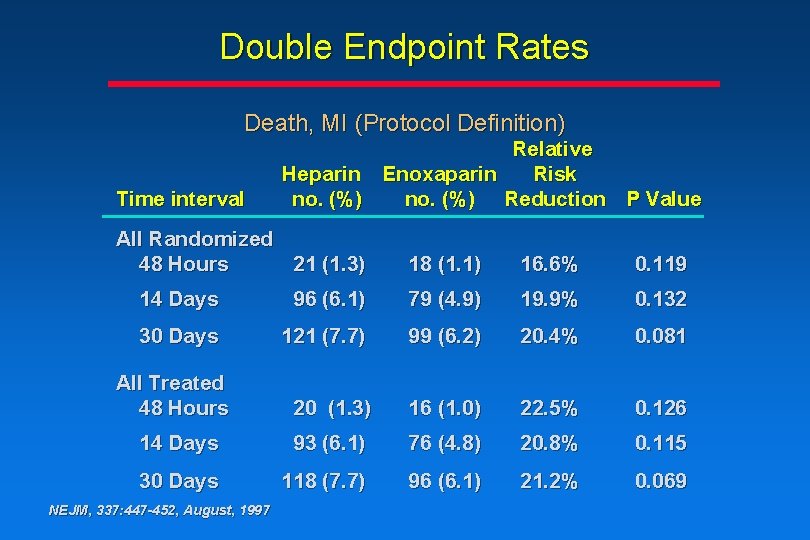
Double Endpoint Rates Death, MI (Protocol Definition) Time interval Relative Heparin Enoxaparin Risk no. (%) Reduction P Value All Randomized 48 Hours 21 (1. 3) 18 (1. 1) 16. 6% 0. 119 14 Days 96 (6. 1) 79 (4. 9) 19. 9% 0. 132 30 Days 121 (7. 7) 99 (6. 2) 20. 4% 0. 081 20 (1. 3) 16 (1. 0) 22. 5% 0. 126 14 Days 93 (6. 1) 76 (4. 8) 20. 8% 0. 115 30 Days 118 (7. 7) 96 (6. 1) 21. 2% 0. 069 All Treated 48 Hours NEJM, 337: 447 -452, August, 1997

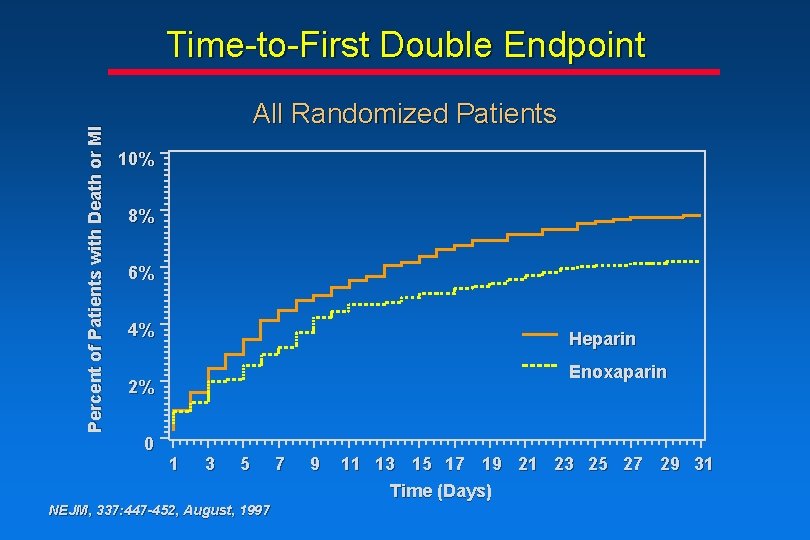
Percent of Patients with Death or MI Time-to-First Double Endpoint All Randomized Patients 10% 8% 6% 4% Heparin Enoxaparin 2% 0 1 3 5 NEJM, 337: 447 -452, August, 1997 7 9 11 13 15 17 19 21 23 25 27 29 31 Time (Days)

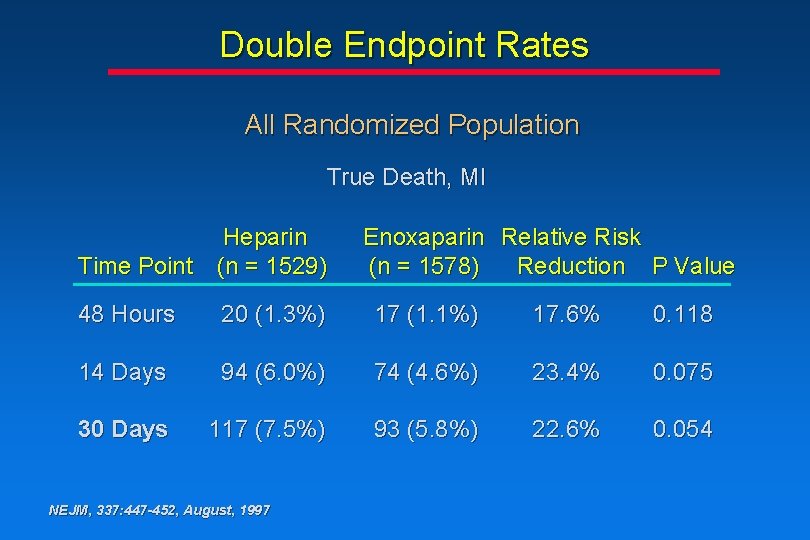
Double Endpoint Rates All Randomized Population True Death, MI Heparin Time Point (n = 1529) Enoxaparin Relative Risk (n = 1578) Reduction P Value 48 Hours 20 (1. 3%) 17 (1. 1%) 17. 6% 0. 118 14 Days 94 (6. 0%) 74 (4. 6%) 23. 4% 0. 075 30 Days 117 (7. 5%) 93 (5. 8%) 22. 6% 0. 054 NEJM, 337: 447 -452, August, 1997

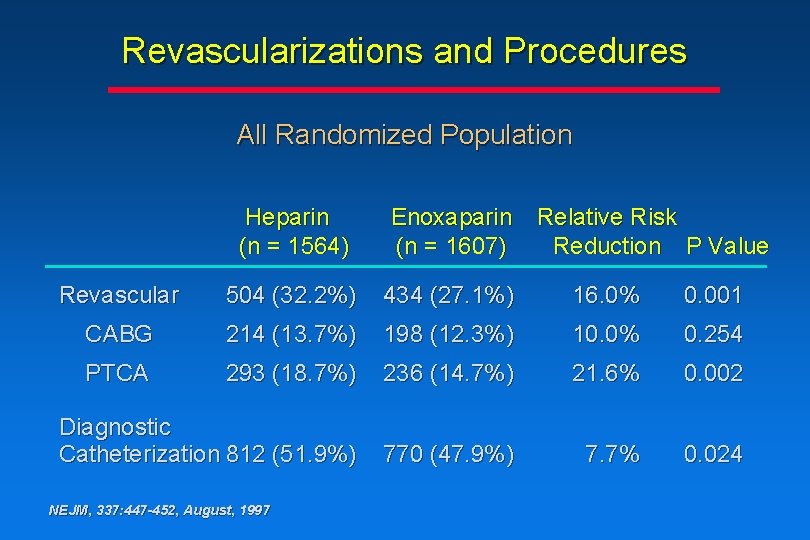
Revascularizations and Procedures All Randomized Population Heparin (n = 1564) Enoxaparin (n = 1607) Revascular 504 (32. 2%) 434 (27. 1%) 16. 0% 0. 001 CABG 214 (13. 7%) 198 (12. 3%) 10. 0% 0. 254 PTCA 293 (18. 7%) 236 (14. 7%) 21. 6% 0. 002 Diagnostic Catheterization 812 (51. 9%) 770 (47. 9%) 7. 7% 0. 024 NEJM, 337: 447 -452, August, 1997 Relative Risk Reduction P Value

Odds Ratio Effect of Baseline Characteristic on Triple Endpoint Favors Unfr. Heparin Favors Enoxaparin Overall Result Male Female Age-1 st Tertile Age-2 nd Tertile Age-3 rd Tertile ECG Changes No ECG Changes ST Depression No ST Depression T Wave Inversion No T Wave Inversion Diabetes Mellitus No Diabetes Mellitus Prior Aspirin Use No Prior Aspirin Use e ** -2 e ** -1 NEJM, 337: 447 -452, August, 1997 e ** 0 Log (Odds Ratio) e ** 1 e ** 2

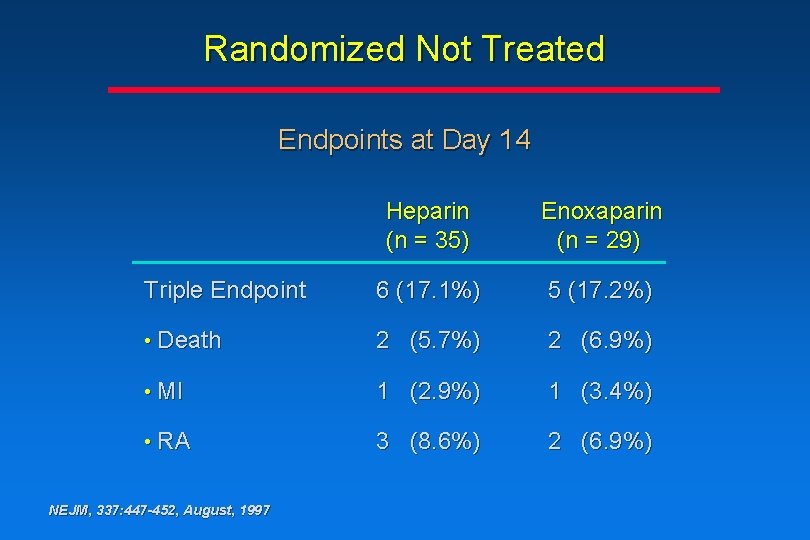
Randomized Not Treated Endpoints at Day 14 Heparin (n = 35) Enoxaparin (n = 29) Triple Endpoint 6 (17. 1%) 5 (17. 2%) • Death 2 (5. 7%) 2 (6. 9%) • MI 1 (2. 9%) 1 (3. 4%) • RA 3 (8. 6%) 2 (6. 9%) NEJM, 337: 447 -452, August, 1997

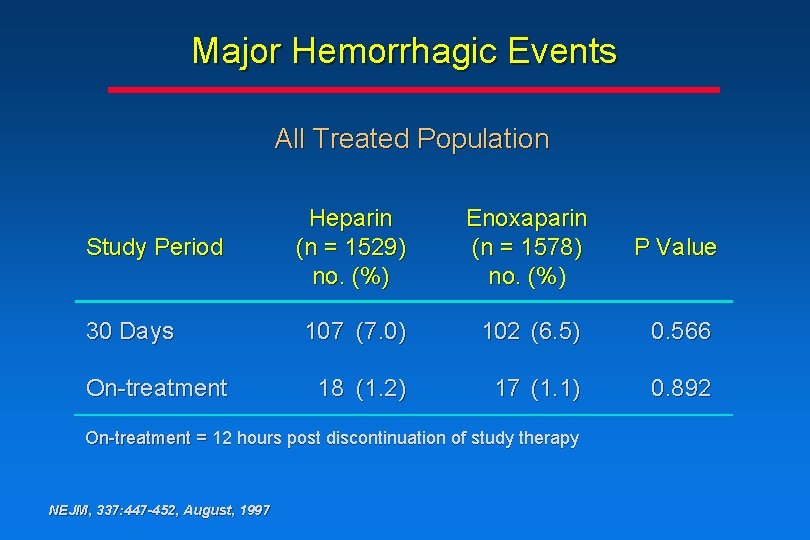
Major Hemorrhagic Events All Treated Population Study Period 30 Days On-treatment Heparin (n = 1529) no. (%) Enoxaparin (n = 1578) no. (%) P Value 107 (7. 0) 102 (6. 5) 0. 566 18 (1. 2) 17 (1. 1) 0. 892 On-treatment = 12 hours post discontinuation of study therapy NEJM, 337: 447 -452, August, 1997

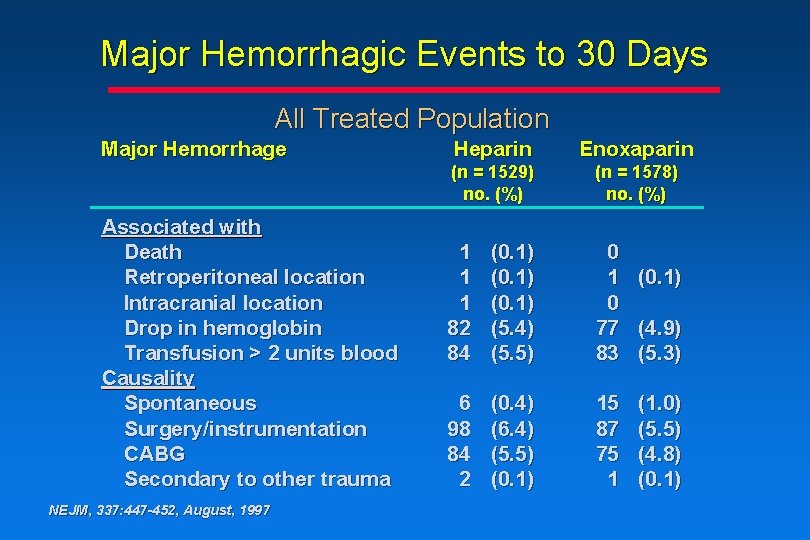
Major Hemorrhagic Events to 30 Days All Treated Population Major Hemorrhage Associated with Death Retroperitoneal location Intracranial location Drop in hemoglobin Transfusion > 2 units blood Causality Spontaneous Surgery/instrumentation CABG Secondary to other trauma NEJM, 337: 447 -452, August, 1997 Heparin Enoxaparin (n = 1529) no. (%) (n = 1578) no. (%) 1 1 1 82 84 (0. 1) (5. 4) (5. 5) 0 1 (0. 1) 0 77 (4. 9) 83 (5. 3) 6 98 84 2 (0. 4) (6. 4) (5. 5) (0. 1) 15 87 75 1 (1. 0) (5. 5) (4. 8) (0. 1)

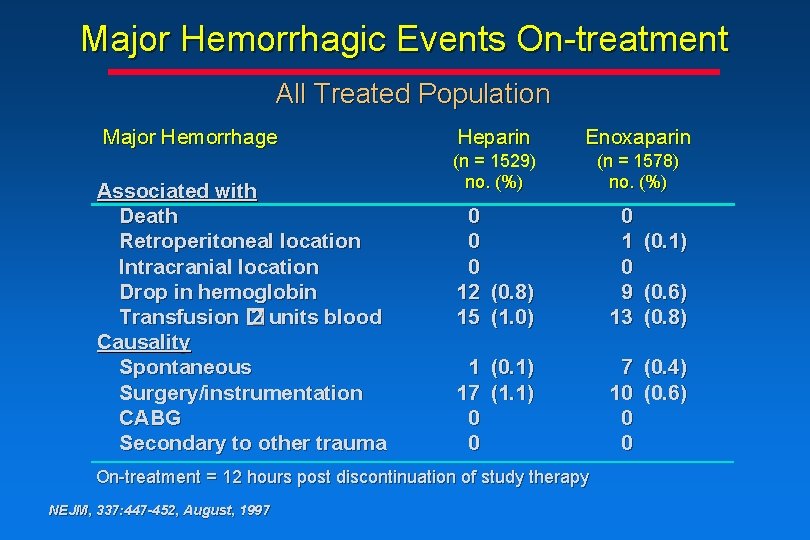
Major Hemorrhagic Events On-treatment All Treated Population Major Hemorrhage Associated with Death Retroperitoneal location Intracranial location Drop in hemoglobin Transfusion � 2 units blood Causality Spontaneous Surgery/instrumentation CABG Secondary to other trauma Heparin Enoxaparin (n = 1529) no. (%) (n = 1578) no. (%) 0 0 0 12 (0. 8) 15 (1. 0) 0 1 0 9 13 1 (0. 1) 17 (1. 1) 0 0 7 (0. 4) 10 (0. 6) 0 0 On-treatment = 12 hours post discontinuation of study therapy NEJM, 337: 447 -452, August, 1997 (0. 1) (0. 6) (0. 8)

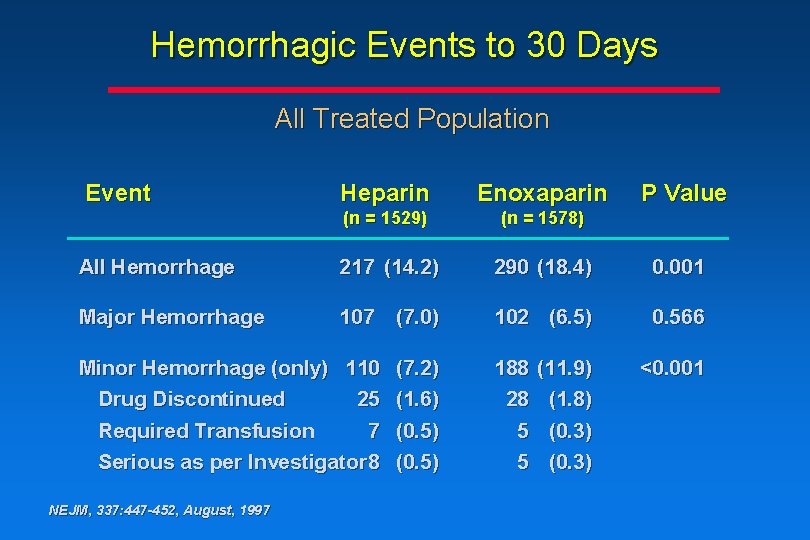
Hemorrhagic Events to 30 Days All Treated Population Event Heparin Enoxaparin (n = 1529) (n = 1578) All Hemorrhage 217 (14. 2) 290 (18. 4) 0. 001 Major Hemorrhage 107 (7. 0) 102 (6. 5) 0. 566 Minor Hemorrhage (only) 110 Drug Discontinued 25 Required Transfusion 7 Serious as per Investigator 8 NEJM, 337: 447 -452, August, 1997 (7. 2) (1. 6) (0. 5) 188 28 5 5 (11. 9) (1. 8) (0. 3) P Value <0. 001

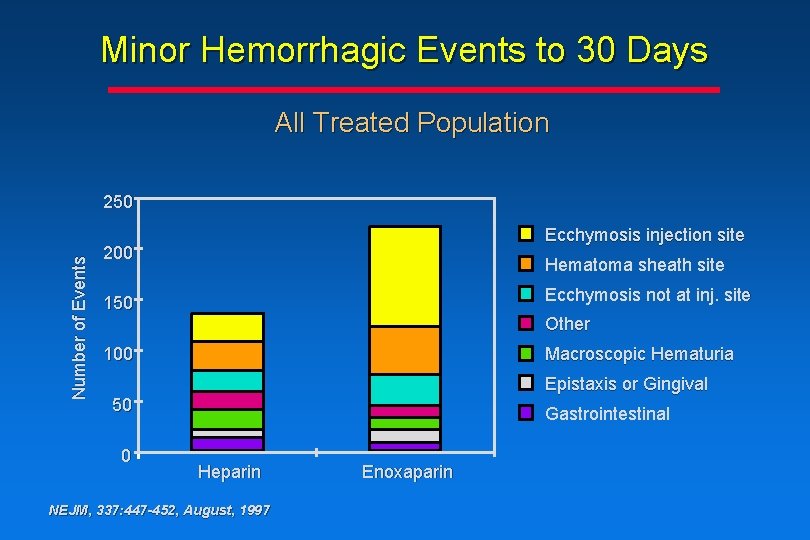
Minor Hemorrhagic Events to 30 Days All Treated Population Number of Events 250 Ecchymosis injection site 200 Hematoma sheath site Ecchymosis not at inj. site 150 Other 100 Macroscopic Hematuria Epistaxis or Gingival 50 0 Gastrointestinal Heparin NEJM, 337: 447 -452, August, 1997 Enoxaparin

Conclusions • At 14 days, the risk of death, myocardial infarction or recurrent angina was significantly lower in patients assigned to enoxaparin compared to heparin (P= 0. 019) • When a more focused definition of recurrent angina is used (angina prompting revascularization), there remains a significant benefit (P=0. 004) • This significant reduction is sustained through 30 days NEJM, 337: 447 -452, August, 1997

Conclusions • The secondary composite endpoint of death or MI demonstrated a strong trend toward fewer events in the enoxaparin group at 30 days NEJM, 337: 447 -452, August, 1997

Conclusions • The rate of major hemorrhagic events associated with enoxaparin treatment vs heparin treatment is comparable • There is an increase in the rate of minor hemorrhagic events associated with enoxaparin treatment vs heparin treatment, due mainly to angiography sheath-site and medication injectionsite ecchymosis or hematoma NEJM, 337: 447 -452, August, 1997

Conclusions • Resource utilization consistently favored enoxaparin as demonstrated by a reduction in the number of diagnostic catheterizations and the number of revascularizations during the 30 -day period NEJM, 337: 447 -452, August, 1997