The Equilibrium Law and Constant Section 7 2

The Equilibrium Law and Constant Section 7. 2
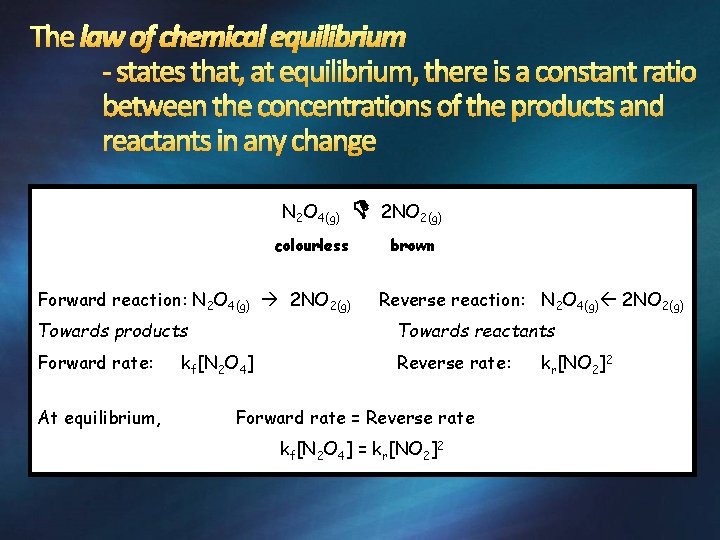
N 2 O 4(g) colourless Forward reaction: N 2 O 4(g) 2 NO 2(g) brown Reverse reaction: N 2 O 4(g) 2 NO 2(g) Towards products Towards reactants Forward rate: Reverse rate: At equilibrium, kf[N 2 O 4] Forward rate = Reverse rate kf[N 2 O 4] = kr[NO 2]2
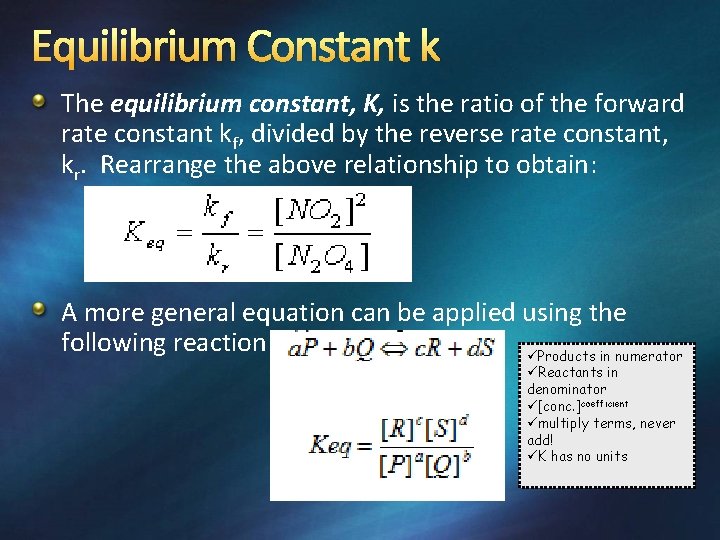
Equilibrium Constant k The equilibrium constant, K, is the ratio of the forward rate constant kf, divided by the reverse rate constant, kr. Rearrange the above relationship to obtain: A more general equation can be applied using the following reaction üProducts in numerator üReactants in denominator ü[conc. ]coefficient ümultiply terms, never add! üK has no units
Temperature Change For a given system at equilibrium, the value of the equilibrium constant depends only on temperature. Changing the temperature of a reacting mixture changes the rate of the forward and reverse reactions by different amounts, because the forward and reverse reactions have different activation energies, Ea.

Heterogeneous Equilibria Homogeneous Equilibrium A chemical equilibrium system in which all reactants and products are in the same state of matter such as the gas state. Example : 2 CO 2(g) 2 CO(g) + O 2(g) Heterogeneous Equilibrium A chemical equilibrium system in which the reactants and products are present in at least two different states, such as gases and solids. Example: Ca. CO 3(s) Ca. O(s) + CO 2(g)

Heterogeneous Equilibria The Equilibrium position of a heterogeneous equilibrium does not depend on the quantities of pure solids or liquids because their concentrations cannot change and are therefore constant. The equilibrium law equation therefore becomes: K = [CO 2(g)] If pure solids or pure liquids are involved in a chemical equilibrium system, their concentrations are not included in the equilibrium law equation for the reaction system. Only gases and aqueous states.

Magnitude of k when K› 1, products are favoured. The equilibrium lies far to the right when K≈1, there approximately equal concentrations of reactants and products at equilibrium when K‹ 1, reactants are favoured. The equilibrium lies far to the left
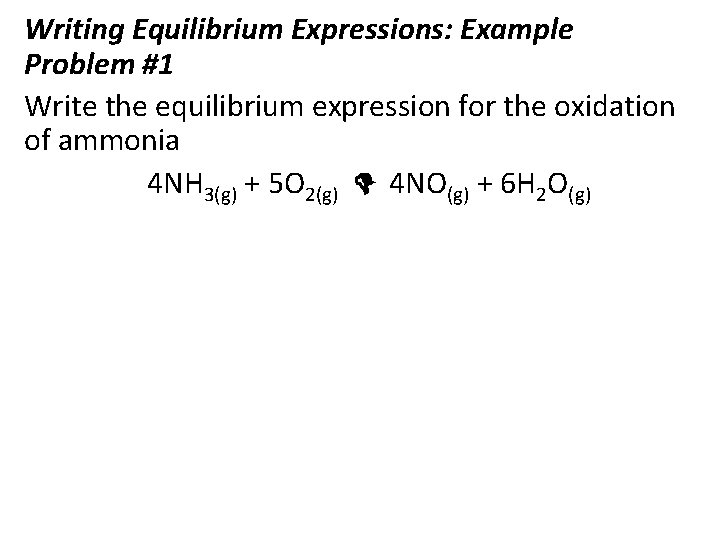
Writing Equilibrium Expressions: Example Problem #1 Write the equilibrium expression for the oxidation of ammonia 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g)

Calculating an Equilibrium Constant: Example Problem #2 Methane, ethyne, and hydrogen form the following equilibrium mixture: 2 CH 4(g) C 2 H 2(g) + 3 H 2(g) While studying this reaction mixture, a chemist analyzed a 4. 0 L sealed flask at 1700 o. C. The chemist found 0. 46 mol of 2 CH 4(g), 0. 65 mol of C 2 H 2(g) and 0. 92 mole of 3 H 2(g). What is the value of K for the reaction at 1700 o. C?

Writing Heterogeneous Expressions: Example Problem #3 Solid phosphorous pentachloride, PCl 5(s), can undergo a reversible reaction to form liquid phosphorous trichloride, PCl 3(l) and chlorine gas, Cl 2(g). Write the equilibrium law expression for this heterogeneous equilibrium.

Temperature and Extent of Reaction: Example Problem #4 Consider the reaction of carbon monoxide and chlorine to form phosgene, COCl 2(g). At 870 K, the value of Kc is 0. 20. At 370 K, the value of Kc is 4. 6 X 107. Based on only the values of K, is the production of COCl 2(g) more favourable at the higher or lower temperature?

Homework Answer Practice Problems #1 -3 p. 431, #1 -3 p. 434, #1 -3, 6 abc
- Slides: 12