The Epidemic of Heart Failure Royal College of











- Slides: 45

The Epidemic of Heart Failure Royal College of Physicians Regional Update in Medicine 2 nd October 2017 Dr Andrew Ludman Cardiology Consultant Royal Devon & Exeter NHS Foundation Trust University of Exeter Medical School a. ludman@nhs. net

Declarations • Support for educational events: Astra Zenaca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Pfizer, Sanofi, Servier, Shire • Speaker fees: Novartis, Sanofi, Astra Zenaca. • Grant for HF service support: Servier


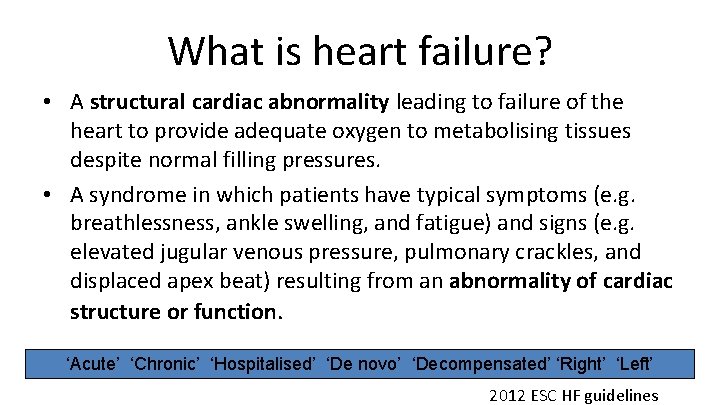
What is heart failure? • A structural cardiac abnormality leading to failure of the heart to provide adequate oxygen to metabolising tissues despite normal filling pressures. • A syndrome in which patients have typical symptoms (e. g. breathlessness, ankle swelling, and fatigue) and signs (e. g. elevated jugular venous pressure, pulmonary crackles, and displaced apex beat) resulting from an abnormality of cardiac structure or function. ‘Acute’ ‘Chronic’ ‘Hospitalised’ ‘De novo’ ‘Decompensated’ ‘Right’ ‘Left’ 2012 ESC HF guidelines

Epidemic of Heart Failure? Or just hyperbole?

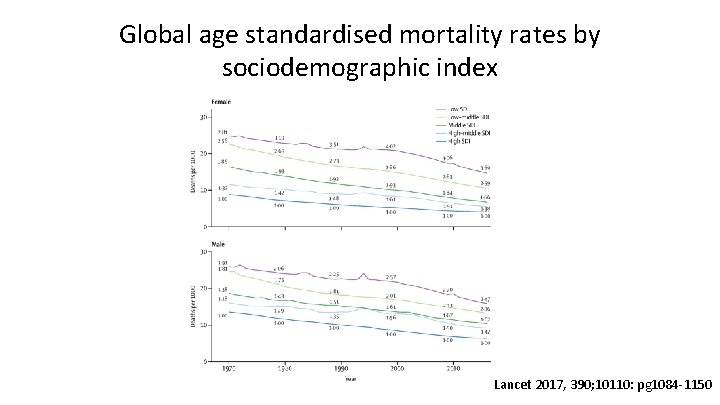
Global age standardised mortality rates by sociodemographic index Lancet 2017, 390; 10110: pg 1084 -1150

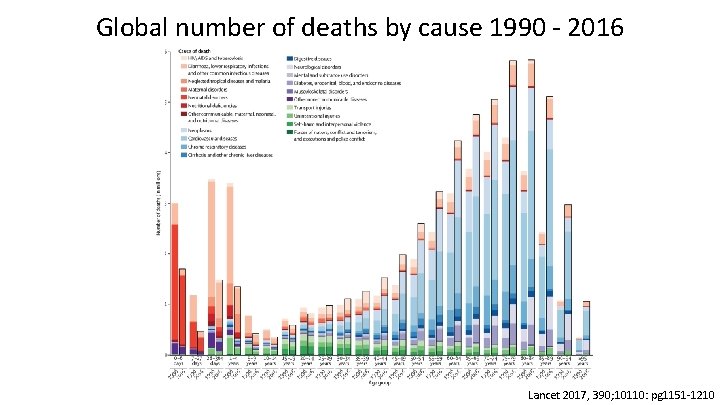
Global number of deaths by cause 1990 - 2016 Lancet 2017, 390; 10110: pg 1151 -1210

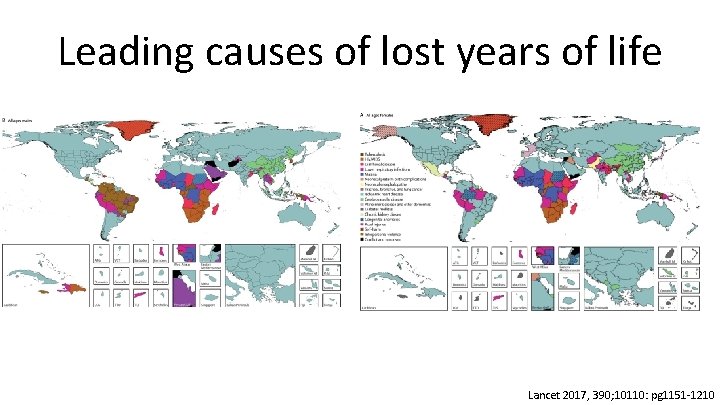
Leading causes of lost years of life Lancet 2017, 390; 10110: pg 1151 -1210

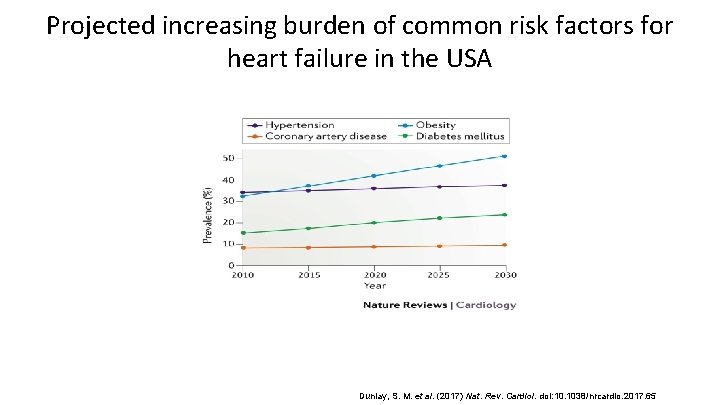
Projected increasing burden of common risk factors for heart failure in the USA Dunlay, S. M. et al. (2017) Nat. Rev. Cardiol. doi: 10. 1038/nrcardio. 2017. 65

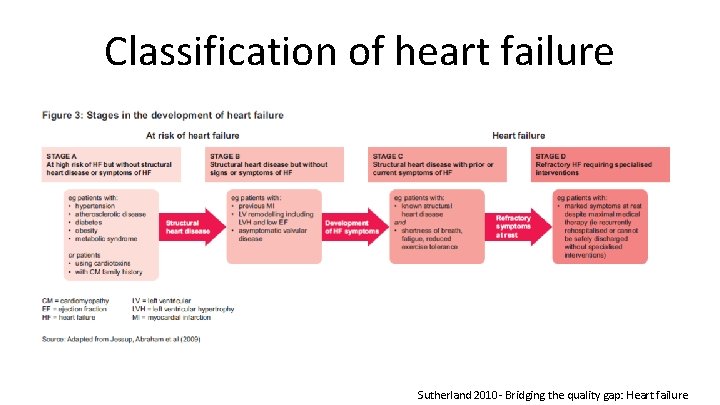
Classification of heart failure Sutherland 2010 - Bridging the quality gap: Heart failure

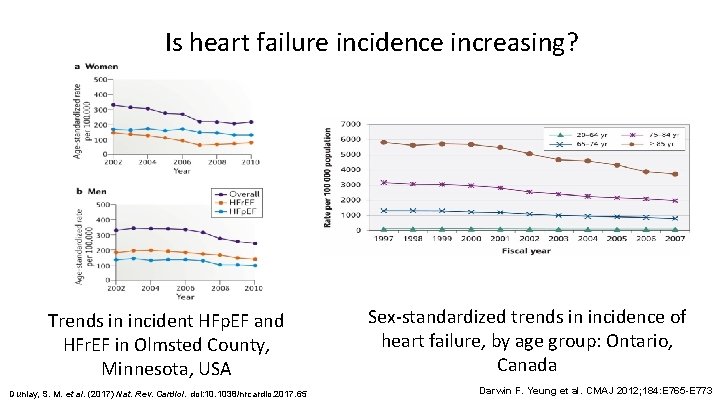
Is heart failure incidence increasing? Trends in incident HFp. EF and HFr. EF in Olmsted County, Minnesota, USA Dunlay, S. M. et al. (2017) Nat. Rev. Cardiol. doi: 10. 1038/nrcardio. 2017. 65 Sex-standardized trends in incidence of heart failure, by age group: Ontario, Canada Darwin F. Yeung et al. CMAJ 2012; 184: E 765 -E 773

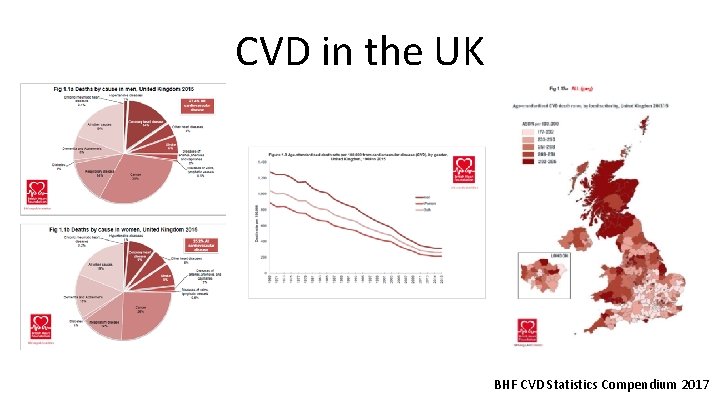
CVD in the UK BHF CVD Statistics Compendium 2017

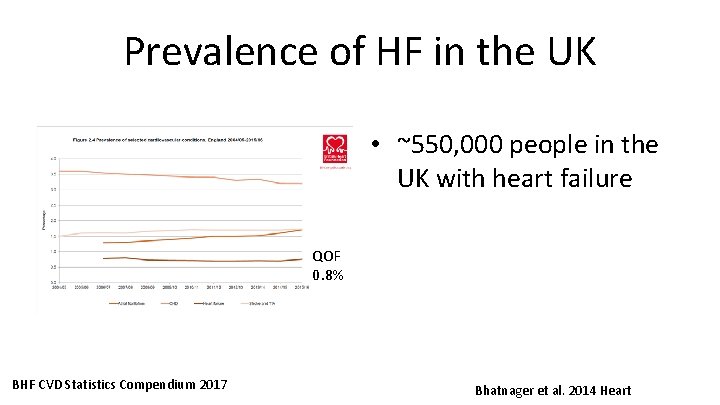
Prevalence of HF in the UK • ~550, 000 people in the UK with heart failure QOF 0. 8% BHF CVD Statistics Compendium 2017 Bhatnager et al. 2014 Heart

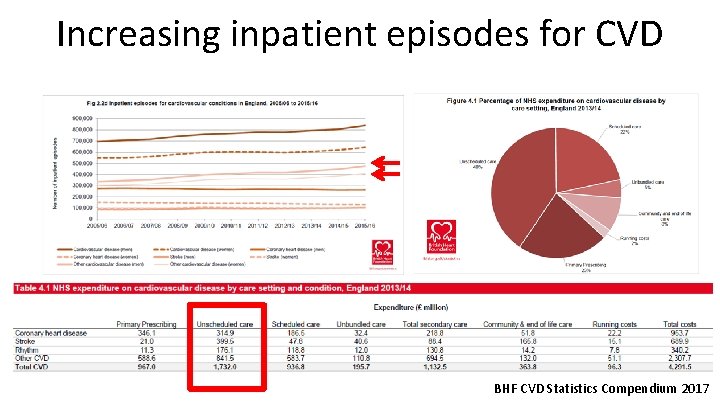
Increasing inpatient episodes for CVD BHF CVD Statistics Compendium 2017

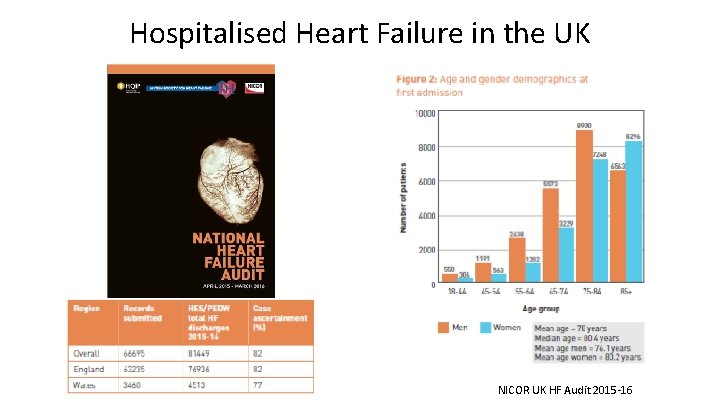
Hospitalised Heart Failure in the UK NICOR UK HF Audit 2015 -16

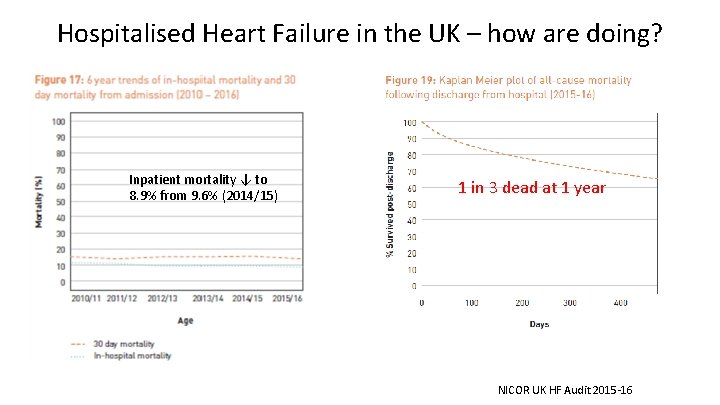
Hospitalised Heart Failure in the UK – how are doing? Inpatient mortality ↓ to 8. 9% from 9. 6% (2014/15) 1 in 3 dead at 1 year NICOR UK HF Audit 2015 -16

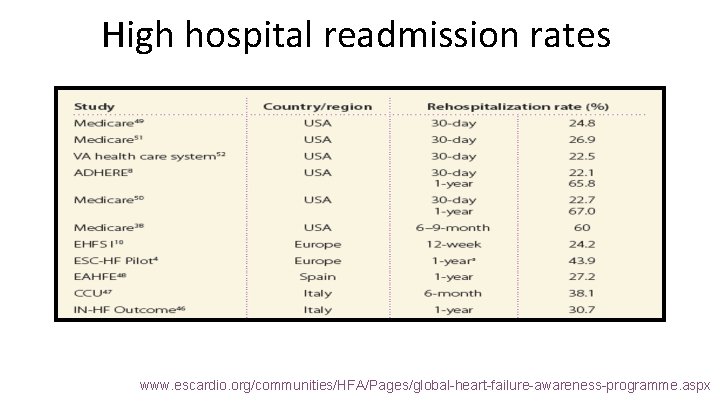
High hospital readmission rates www. escardio. org/communities/HFA/Pages/global-heart-failure-awareness-programme. aspx

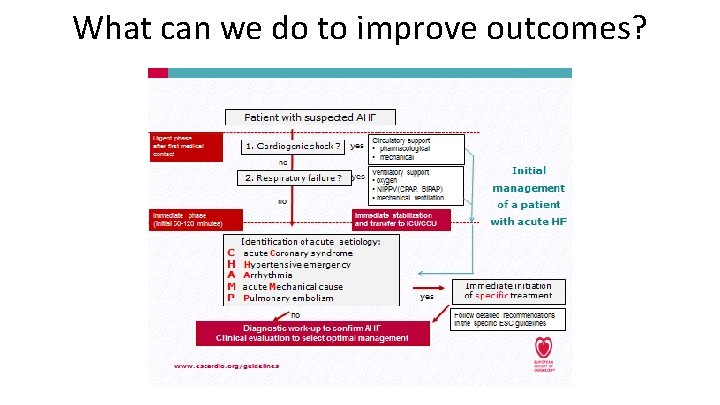
What can we do to improve outcomes?

What can we do to improve outcomes? NICE Quality Standard on Acute HF. 2015. Available at: https: //www. nice. org. uk/guidance/qs 103 Last accessed: Sept 2017.

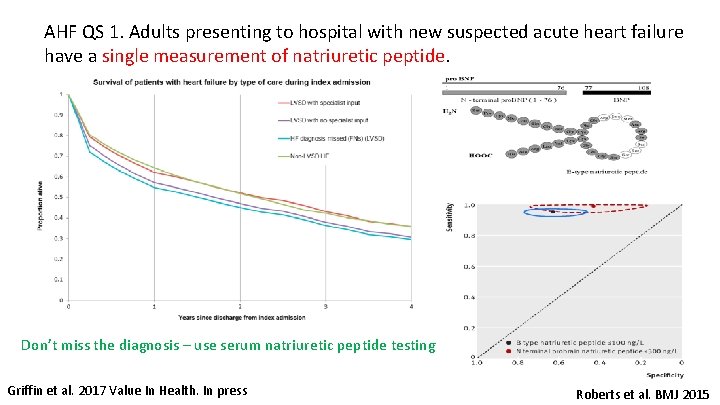
AHF QS 1. Adults presenting to hospital with new suspected acute heart failure have a single measurement of natriuretic peptide. Don’t miss the diagnosis – use serum natriuretic peptide testing Griffin et al. 2017 Value In Health. In press Roberts et al. BMJ 2015

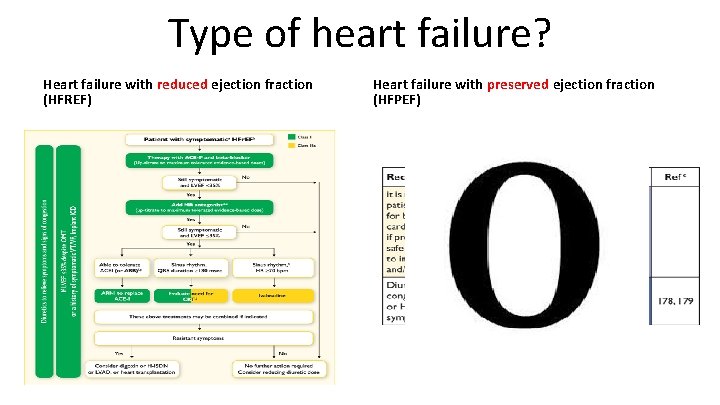
AHF QS 2. Adults admitted to hospital with new suspected acute heart failure and raised natriuretic peptide levels have a transthoracic Doppler 2 D echocardiogram within 48 hours of admission. • Heart failure with reduced ejection fraction (HFREF) • Heart failure with preserved ejection fraction (HFPEF)

Type of heart failure? Heart failure with reduced ejection fraction (HFREF) Heart failure with preserved ejection fraction (HFPEF)

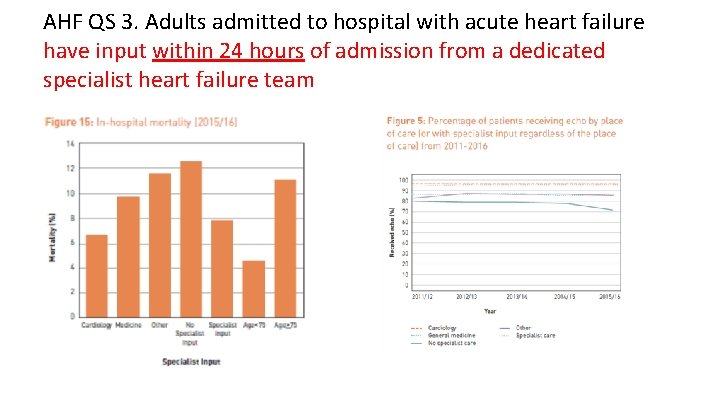
AHF QS 3. Adults admitted to hospital with acute heart failure have input within 24 hours of admission from a dedicated specialist heart failure team

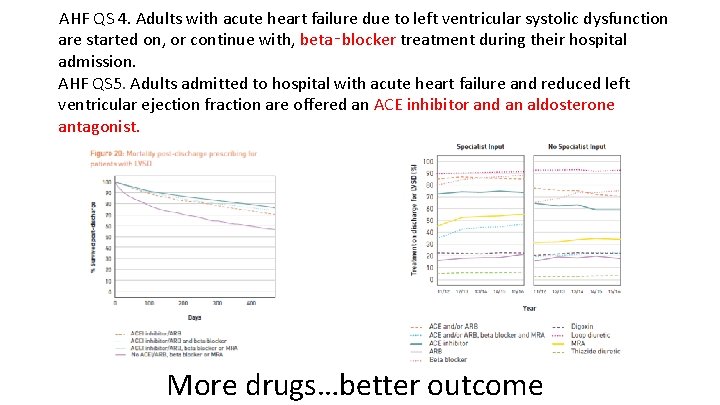
AHF QS 4. Adults with acute heart failure due to left ventricular systolic dysfunction are started on, or continue with, beta‑blocker treatment during their hospital admission. AHF QS 5. Adults admitted to hospital with acute heart failure and reduced left ventricular ejection fraction are offered an ACE inhibitor and an aldosterone antagonist. More drugs…better outcome

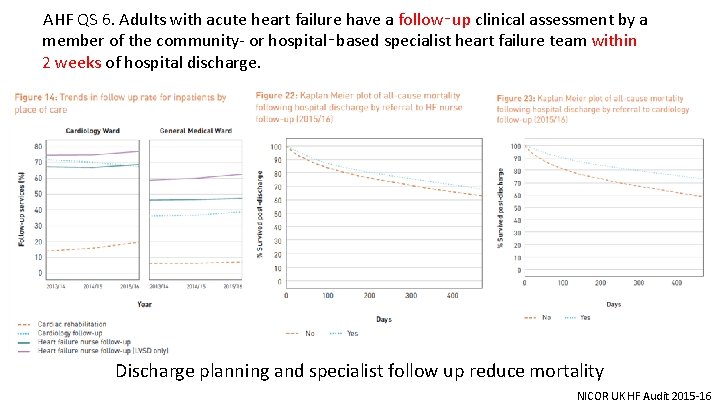
AHF QS 6. Adults with acute heart failure have a follow‑up clinical assessment by a member of the community- or hospital‑based specialist heart failure team within 2 weeks of hospital discharge. Discharge planning and specialist follow up reduce mortality NICOR UK HF Audit 2015 -16

Diuretics – an old dog?

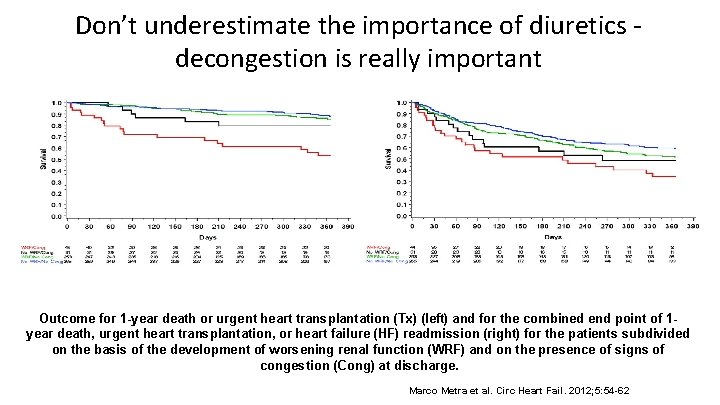
Don’t underestimate the importance of diuretics - decongestion is really important Outcome for 1 -year death or urgent heart transplantation (Tx) (left) and for the combined end point of 1 year death, urgent heart transplantation, or heart failure (HF) readmission (right) for the patients subdivided on the basis of the development of worsening renal function (WRF) and on the presence of signs of congestion (Cong) at discharge. Marco Metra et al. Circ Heart Fail. 2012; 5: 54 -62

A new trick?

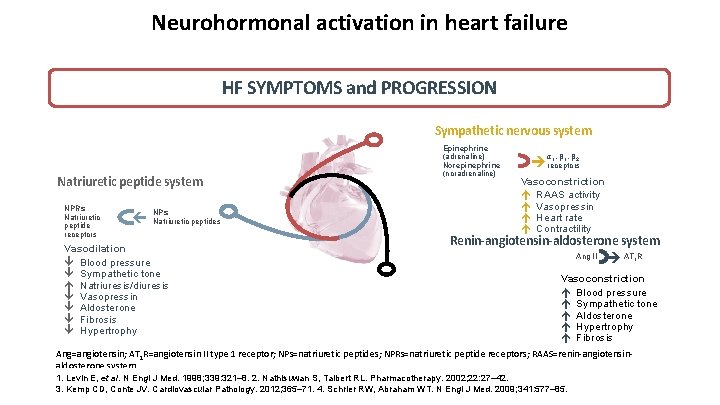
Neurohormonal activation in heart failure HF SYMPTOMS and PROGRESSION Sympathetic nervous system NPs ç Natriuretic peptides Vasodilation Blood pressure Sympathetic tone Natriuresis/diuresis Vasopressin Aldosterone Fibrosis Hypertrophy α 1, β 2 receptors Vasoconstriction RAAS activity Vasopressin Heart rate Contractility Renin-angiotensin-aldosterone system Ang II ç NPRs Natriuretic peptide receptors ç Natriuretic peptide system Epinephrine (adrenaline) Norepinephrine (noradrenaline) AT 1 R Vasoconstriction Blood pressure Sympathetic tone Aldosterone Hypertrophy Fibrosis Ang=angiotensin; AT 1 R=angiotensin II type 1 receptor; NPs=natriuretic peptides; NPRs=natriuretic peptide receptors; RAAS=renin-angiotensinaldosterone system. 1. Levin E, et al. N Engl J Med. 1998; 339: 321– 8. 2. Nathisuwan S, Talbert RL. Pharmacotherapy. 2002; 22: 27– 42. 3. Kemp CD, Conte JV. Cardiovascular Pathology. 2012; 365– 71. 4. Schrier RW, Abraham WT. N Engl J Med. 2009; 341: 577– 85.

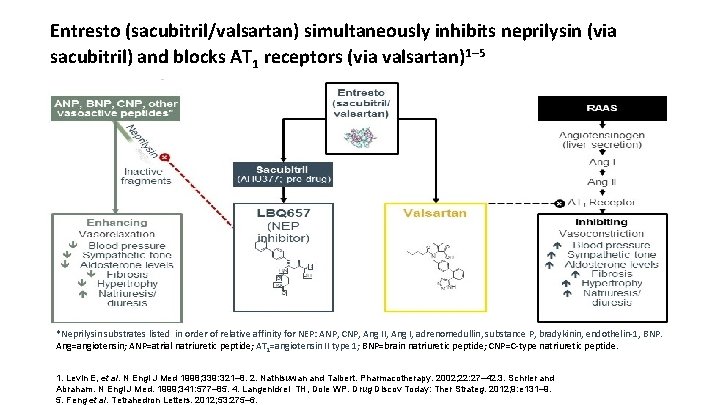
Entresto (sacubitril/valsartan) simultaneously inhibits neprilysin (via sacubitril) and blocks AT 1 receptors (via valsartan)1– 5 *Neprilysin substrates listed in order of relative affinity for NEP: ANP, CNP, Ang II, Ang I, adrenomedullin, substance P, bradykinin, endothelin-1, BNP. Ang=angiotensin; ANP=atrial natriuretic peptide; AT 1=angiotensin II type 1; BNP=brain natriuretic peptide; CNP=C-type natriuretic peptide. 1. Levin E, et al. N Engl J Med 1998; 339: 321– 8. 2. Nathisuwan and Talbert. Pharmacotherapy. 2002; 22: 27– 42. 3. Schrier and Abraham. N Engl J Med. 1999; 341: 577– 85. 4. Langenickel TH, Dole WP. Drug Discov Today: Ther Strateg. 2012; 9: e 131– 9. 5. Feng et al. Tetrahedron Letters. 2012; 53: 275– 6.

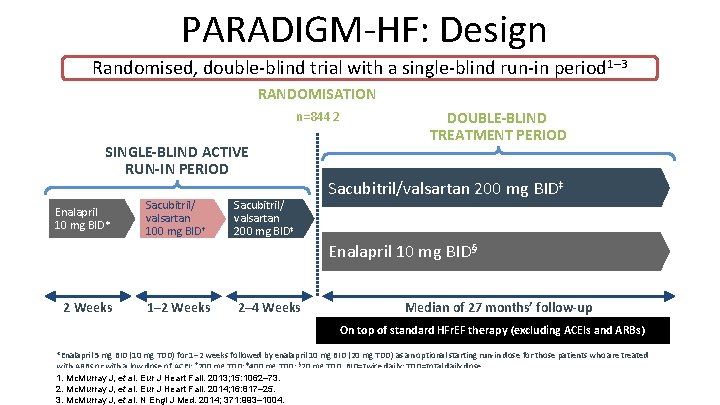
PARADIGM-HF: Design Randomised, double-blind trial with a single-blind run-in period 1– 3 RANDOMISATION n=8442 SINGLE-BLIND ACTIVE RUN-IN PERIOD Enalapril 10 mg BID* Sacubitril/ valsartan 100 mg BID† Sacubitril/ valsartan 200 mg BID‡ DOUBLE-BLIND TREATMENT PERIOD Sacubitril/valsartan 200 mg BID‡ Enalapril 10 mg BID§ 2 Weeks 1– 2 Weeks 2– 4 Weeks Median of 27 months’ follow-up On top of standard HFr. EF therapy (excluding ACEIs and ARBs) *Enalapril 5 mg BID (10 mg TDD) for 1– 2 weeks followed by enalapril 10 mg BID (20 mg TDD) as an optional starting run-in dose for those patients who are treated with ARBs or with a low dose of ACEI; † 200 mg TDD; ‡ 400 mg TDD; § 20 mg TDD. BID=twice daily; TDD=total daily dose. 1. Mc. Murray J, et al. Eur J Heart Fail. 2013; 15: 1062– 73. 2. Mc. Murray J, et al. Eur J Heart Fail. 2014; 16: 817– 25. 3. Mc. Murray J, et al. N Engl J Med. 2014; 371: 993– 1004.

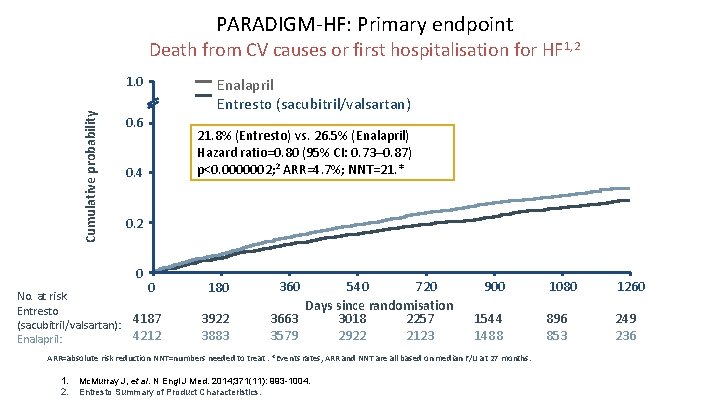
PARADIGM-HF: Primary endpoint Death from CV causes or first hospitalisation for HF 1, 2 Cumulative probability 1. 0 0. 6 21. 8% (Entresto) vs. 26. 5% (Enalapril) Hazard ratio=0. 80 (95% CI: 0. 73– 0. 87) p<0. 0000002; 2 ARR=4. 7%; NNT=21. * 0. 4 0. 2 0 No. at risk Entresto (sacubitril/valsartan): Enalapril: Enalapril Entresto (sacubitril/valsartan) 0 180 4187 4212 3922 3883 360 540 720 Days since randomisation 3663 3018 2257 3579 2922 2123 900 1544 1488 ARR=absolute risk reduction NNT=numbers needed to treat. *Events rates, ARR and NNT are all based on median F/U at 27 months. 1. 2. Mc. Murray J, et al. N Engl J Med. 2014; 371(11): 993 -1004. Entresto Summary of Product Characteristics. 1080 1260 896 853 249 236

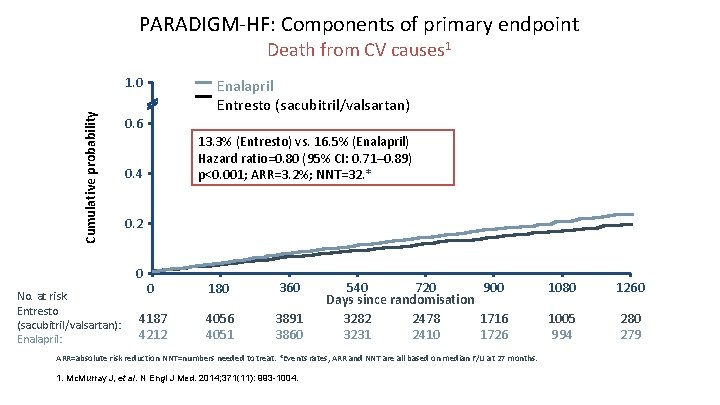
PARADIGM-HF: Components of primary endpoint Death from CV causes 1 Cumulative probability 1. 0 0. 6 13. 3% (Entresto) vs. 16. 5% (Enalapril) Hazard ratio=0. 80 (95% CI: 0. 71– 0. 89) p<0. 001; ARR=3. 2%; NNT=32. * 0. 4 0. 2 0 No. at risk Entresto (sacubitril/valsartan): Enalapril: Enalapril Entresto (sacubitril/valsartan) 0 180 360 4187 4212 4056 4051 3891 3860 540 720 900 Days since randomisation 3282 2478 1716 3231 2410 1726 ARR=absolute risk reduction NNT=numbers needed to treat. *Events rates, ARR and NNT are all based on median F/U at 27 months. 1. Mc. Murray J, et al. N Engl J Med. 2014; 371(11): 993 -1004. 1080 1260 1005 994 280 279

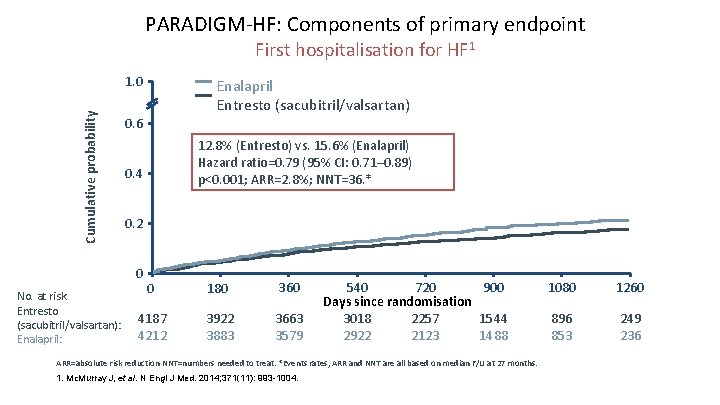
PARADIGM-HF: Components of primary endpoint First hospitalisation for HF 1 Cumulative probability 1. 0 0. 6 12. 8% (Entresto) vs. 15. 6% (Enalapril) Hazard ratio=0. 79 (95% CI: 0. 71– 0. 89) p<0. 001; ARR=2. 8%; NNT=36. * 0. 4 0. 2 0 No. at risk Entresto (sacubitril/valsartan): Enalapril: Enalapril Entresto (sacubitril/valsartan) 0 180 360 4187 4212 3922 3883 3663 3579 540 720 900 Days since randomisation 3018 2257 1544 2922 2123 1488 ARR=absolute risk reduction NNT=numbers needed to treat. *Events rates, ARR and NNT are all based on median F/U at 27 months. 1. Mc. Murray J, et al. N Engl J Med. 2014; 371(11): 993 -1004. 1080 1260 896 853 249 236

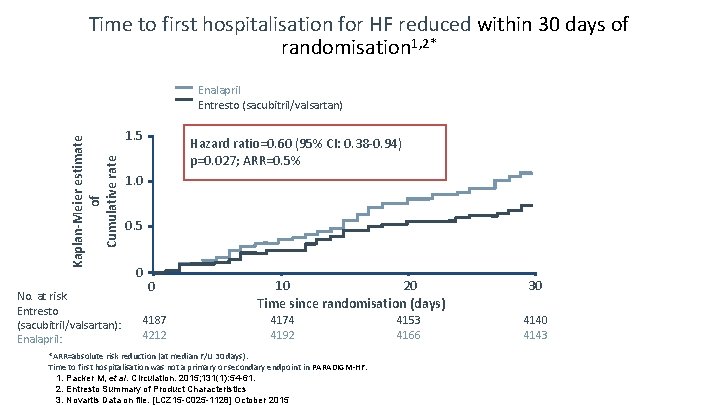
Time to first hospitalisation for HF reduced within 30 days of randomisation 1, 2* Kaplan-Meier estimate of Cumulative rate Enalapril Entresto (sacubitril/valsartan) No. at risk Entresto (sacubitril/valsartan): Enalapril: 1. 5 Hazard ratio=0. 60 (95% CI: 0. 38 -0. 94) p=0. 027; ARR=0. 5% 1. 0 0. 5 0 0 4187 4212 10 20 Time since randomisation (days) 4174 4192 *ARR=absolute risk reduction (at median F/U 30 days). Time to first hospitalisation was not a primary or secondary endpoint in PARADIGM-HF. 1. Packer M, et al. Circulation. 2015; 131(1): 54 -61. 2. Entresto Summary of Product Characteristics 3. Novartis Data on file. [LCZ 15 -C 025 -1128] October 2015 4153 4166 30 4143

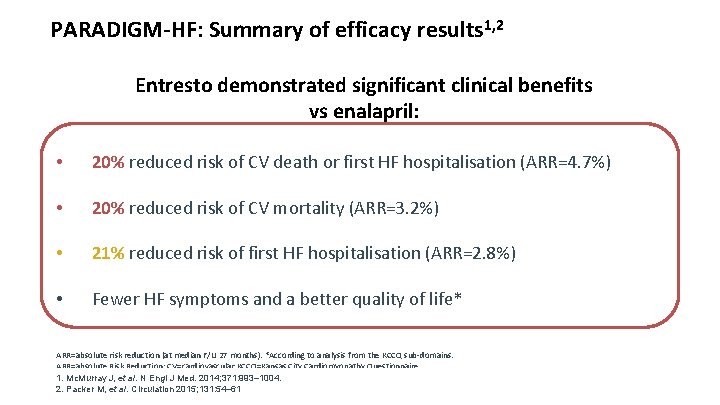
PARADIGM-HF: Summary of efficacy results 1, 2 Entresto demonstrated significant clinical benefits vs enalapril: • 20% reduced risk of CV death or first HF hospitalisation (ARR=4. 7%) • 20% reduced risk of CV mortality (ARR=3. 2%) • 21% reduced risk of first HF hospitalisation (ARR=2. 8%) • Fewer HF symptoms and a better quality of life* ARR=absolute risk reduction (at median F/U 27 months). *According to analysis from the KCCQ sub-domains. ARR=absolute Risk Reduction; CV=cardiovascular KCCQ=Kansas City Cardiomyopathy Questionnaire. 1. Mc. Murray J, et al. N Engl J Med. 2014; 371: 993– 1004. 2. Packer M, et al. Circulation 2015; 131: 54– 61

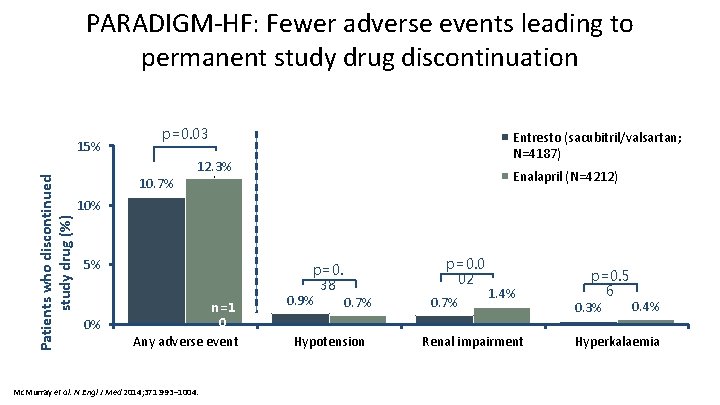
PARADIGM-HF: Fewer adverse events leading to permanent study drug discontinuation Patients who discontinued study drug (%) 15% p=0. 03 10. 7% Entresto (sacubitril/valsartan; N=4187) 12. 3% Enalapril (N=4212) 10% 5% n=1 0 0% Any adverse event Mc. Murray et al. N Engl J Med 2014; 371: 993– 1004. n=5 p=0. 38 0. 9% 0. 7% Hypotension p=0. 0 02 0. 7% 1. 4% Renal impairment p=0. 5 6 0. 4% 0. 3% Hyperkalaemia

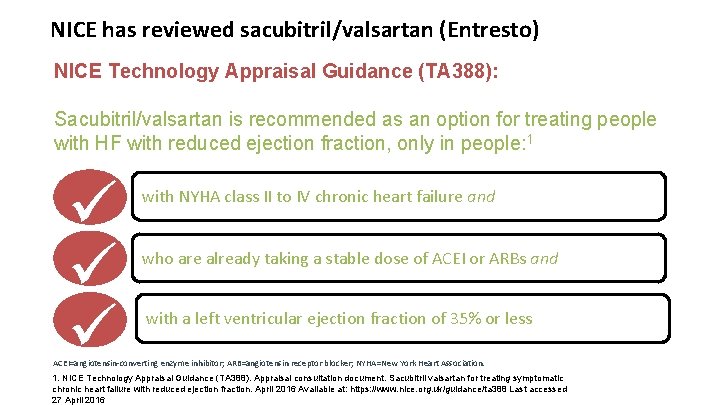
NICE has reviewed sacubitril/valsartan (Entresto) NICE Technology Appraisal Guidance (TA 388): Sacubitril/valsartan is recommended as an option for treating people with HF with reduced ejection fraction, only in people: 1 with NYHA class II to IV chronic heart failure and who are already taking a stable dose of ACEI or ARBs and with a left ventricular ejection fraction of 35% or less ACEI=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; NYHA=New York Heart Association. 1. NICE Technology Appraisal Guidance (TA 388). Appraisal consultation document. Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. April 2016 Available at: https: //www. nice. org. uk/guidance/ta 388 Last accessed 27 April 2016

NICE TAG Recommendation NICE Technology Appraisal Guidance recommends that: Treatment with sacubitril/valsartan should be started by a heart failure specialist with access to a multidisciplinary heart failure team 1 Dose titration and monitoring should be performed by the most appropriate team member as defined in NICE’s guideline on chronic heart failure in adults 2 1. NICE Technology Appraisal Guidance (TA 388). Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. April 2016 Available at: https: //www. nice. org. uk/guidance/ta 388 Last accessed 27 April 2016 2. NICE. Chronic HF Guideline. August 2010 Available at: https: //www. nice. org. uk/guidance/cg 108 Last accessed 27 April 2016

Where next for sacubitril/valsartan? • • • Hospitalised/Acute heart failure Starting directly (no ACE-I) HF PEF Hypertension …. .

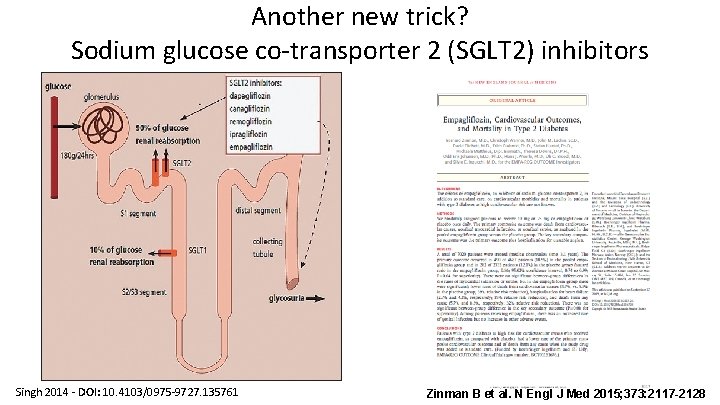
Another new trick? Sodium glucose co-transporter 2 (SGLT 2) inhibitors Singh 2014 - DOI: 10. 4103/0975 -9727. 135761 Zinman B et al. N Engl J Med 2015; 373: 2117 -2128

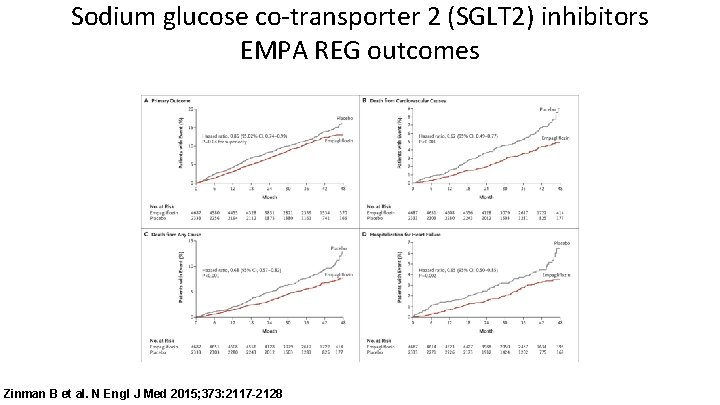
Sodium glucose co-transporter 2 (SGLT 2) inhibitors EMPA REG outcomes Zinman B et al. N Engl J Med 2015; 373: 2117 -2128

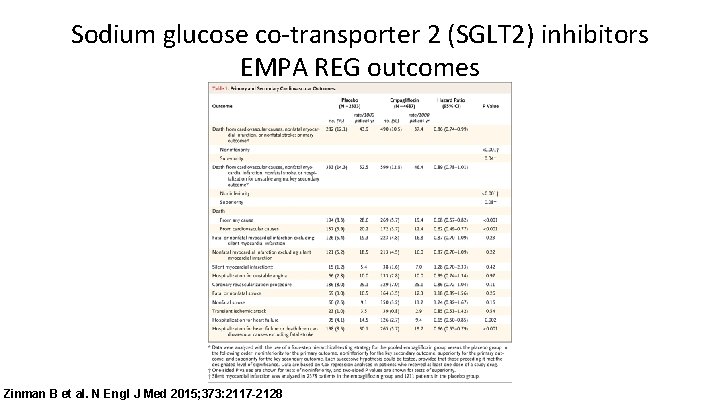
Sodium glucose co-transporter 2 (SGLT 2) inhibitors EMPA REG outcomes Zinman B et al. N Engl J Med 2015; 373: 2117 -2128

Conclusions • Not sure about the HF epidemic yet • Outcomes for hospitalised patients are improving but remain poor • Optimise fluid balance and medication predischarge • Specialist involvement and follow up • Active drug development – watch this space

Additional resources for patients