The EM Spectrum and Radioactive Decay True or










- Slides: 21

The EM Spectrum and Radioactive Decay

• True or False? Sun’s Energy The heat the sun supplies allows for life on earth.

FALSE!!! • The energy the sun supplies allows for life on earth. • The sun does not give out heat – it gives out radiation (which is not hot if measured with a thermometer). • The radiation is absorbed by everything the light hits and makes the molecules of that object move. • Moving molecules create friction, which gives us heat!!!!

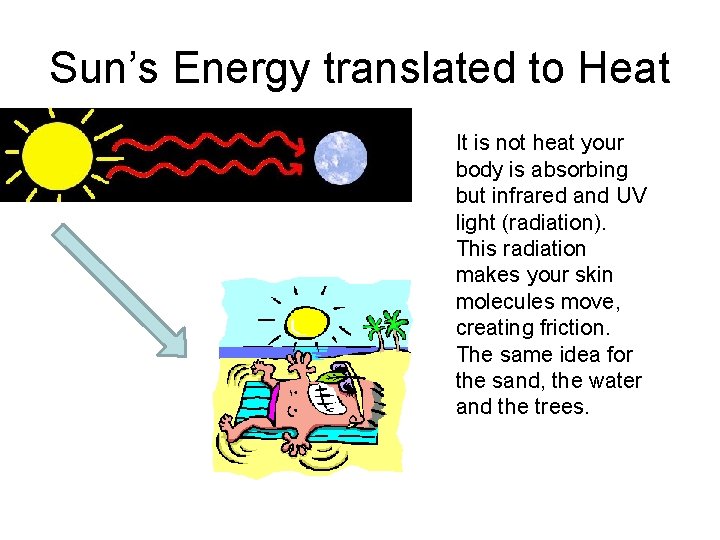
Sun’s Energy translated to Heat It is not heat your body is absorbing but infrared and UV light (radiation). This radiation makes your skin molecules move, creating friction. The same idea for the sand, the water and the trees.

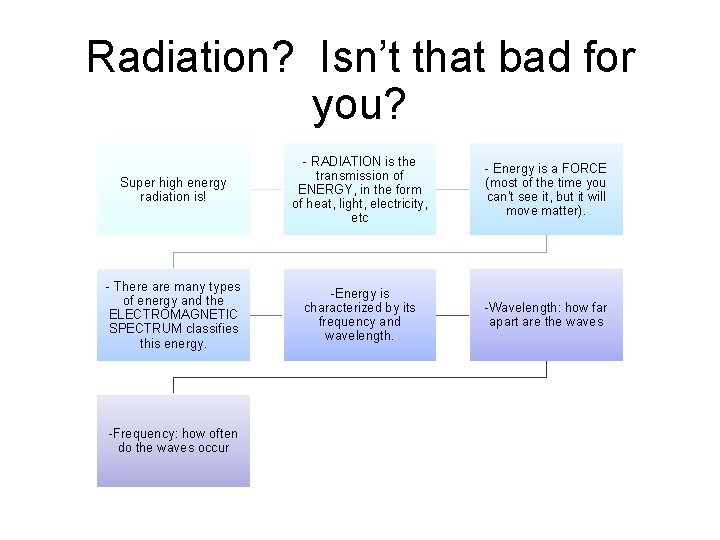
Radiation? Isn’t that bad for you? Super high energy radiation is! - RADIATION is the transmission of ENERGY, in the form of heat, light, electricity, etc - Energy is a FORCE (most of the time you can’t see it, but it will move matter). - There are many types of energy and the ELECTROMAGNETIC SPECTRUM classifies this energy. -Energy is characterized by its frequency and wavelength. -Wavelength: how far apart are the waves -Frequency: how often do the waves occur

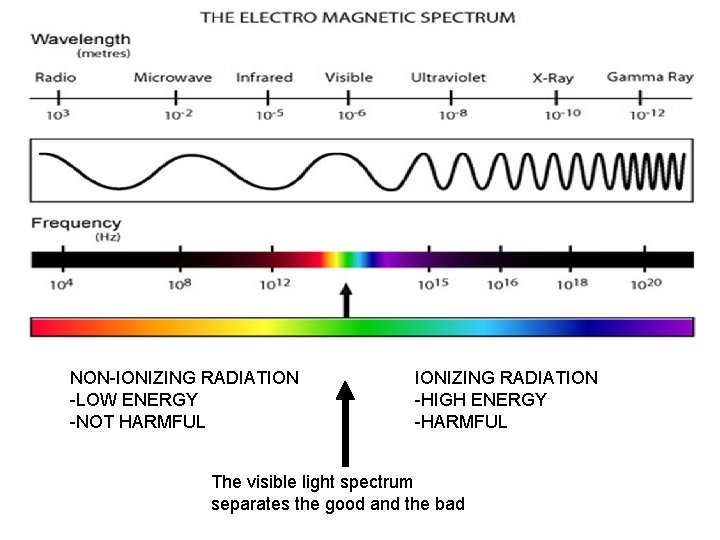
NON-IONIZING RADIATION -LOW ENERGY -NOT HARMFUL IONIZING RADIATION -HIGH ENERGY -HARMFUL The visible light spectrum separates the good and the bad

RADIOAC TIVE DECAY: WHY DOES IT HAPPEN?

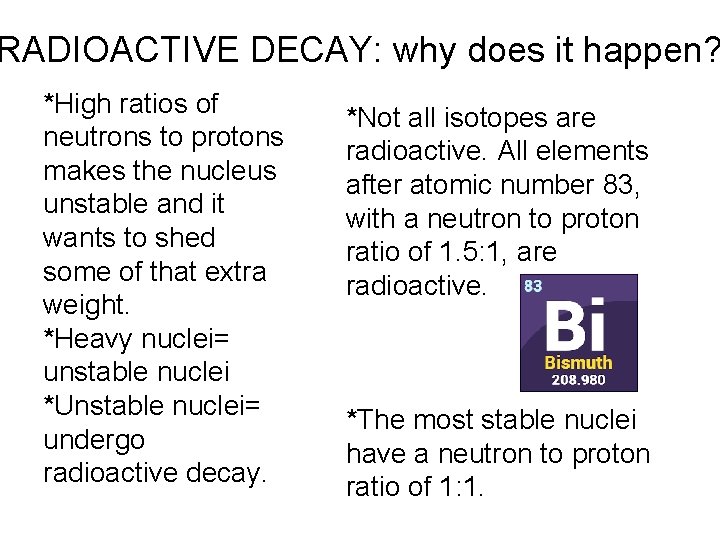
RADIOACTIVE DECAY: why does it happen? *High ratios of neutrons to protons makes the nucleus unstable and it wants to shed some of that extra weight. *Heavy nuclei= unstable nuclei *Unstable nuclei= undergo radioactive decay. *Not all isotopes are radioactive. All elements after atomic number 83, with a neutron to proton ratio of 1. 5: 1, are radioactive. *The most stable nuclei have a neutron to proton ratio of 1: 1.

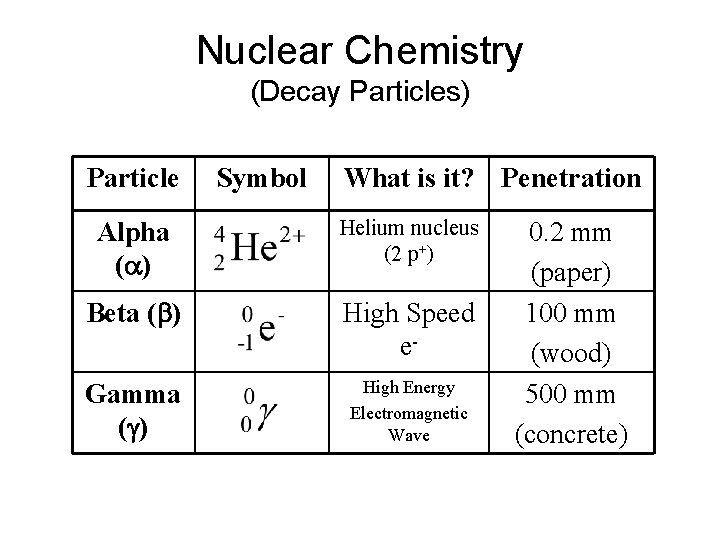
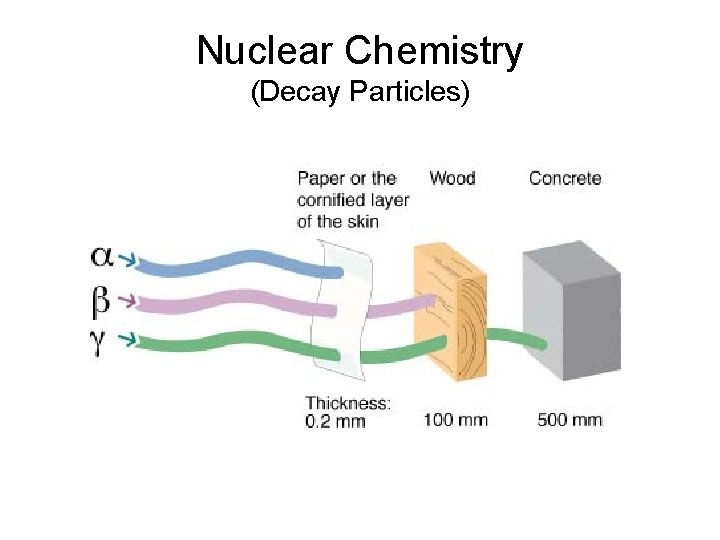
Nuclear Chemistry (Decay Particles) Particle Symbol What is it? Penetration Alpha (a) Helium nucleus (2 p+) Beta (b) High Speed e- Gamma (g) High Energy Electromagnetic Wave 0. 2 mm (paper) 100 mm (wood) 500 mm (concrete)

Nuclear Chemistry (Decay Particles)

Nuclei may eject an alpha particle, which has 2 protons and 2 neutrons, just like a helium nucleus. The alpha particle can be designated by using either of these symbols

Americium-241 is used in smoke detectors. This isotope is unstable and will emit an alpha particle.

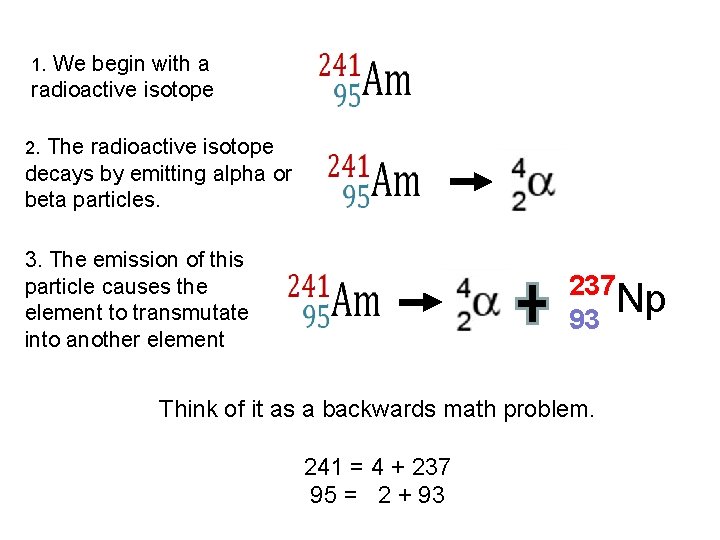
1. We begin with a radioactive isotope 2. The radioactive isotope decays by emitting alpha or beta particles. 3. The emission of this particle causes the element to transmutate into another element 237 Np 93 Think of it as a backwards math problem. 241 = 4 + 237 95 = 2 + 93

• What do we end up with after the isotope radium-226 emits an alpha particle?

The beta particle can be designated by using either of these symbols

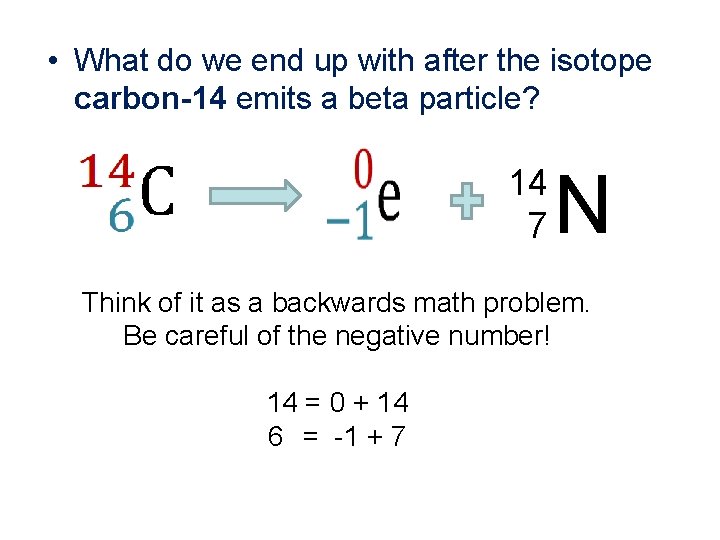
• What do we end up with after the isotope carbon-14 emits a beta particle? 14 7 N Think of it as a backwards math problem. Be careful of the negative number! 14 = 0 + 14 6 = -1 + 7

• Is this an alpha or beta equation? How do you know? ? ?

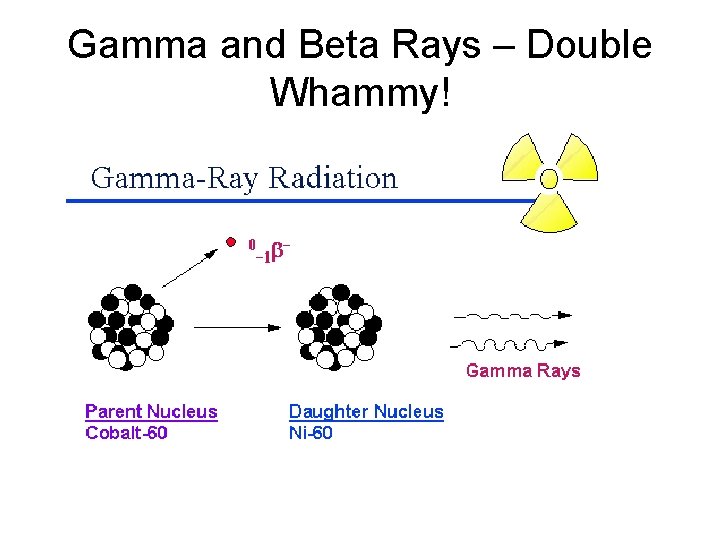
Gamma and Beta Rays – Double Whammy!

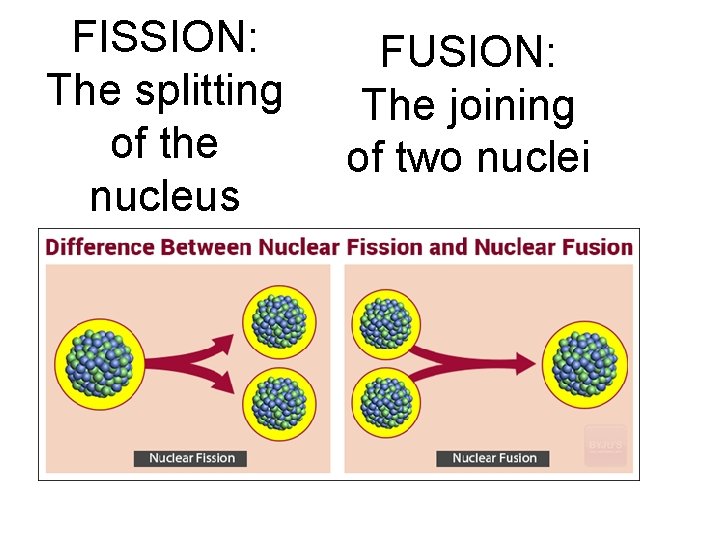
FISSION: The splitting of the nucleus FUSION: The joining of two nuclei

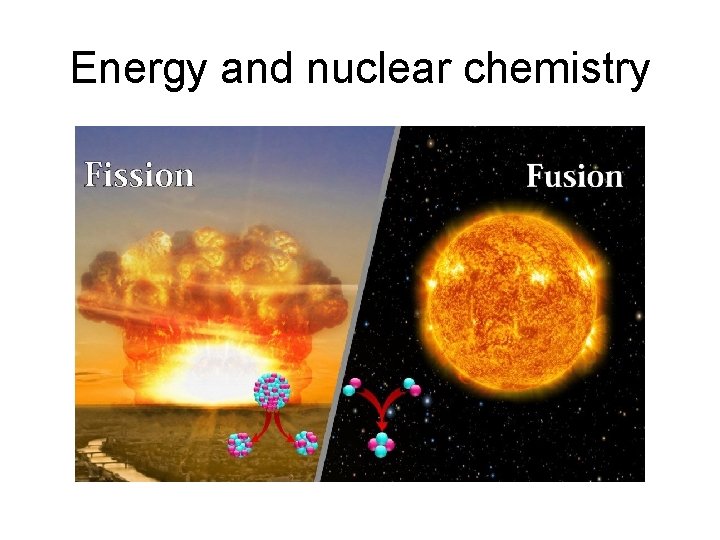
Energy and nuclear chemistry
