The Elements of Group II The Alkaline Earth



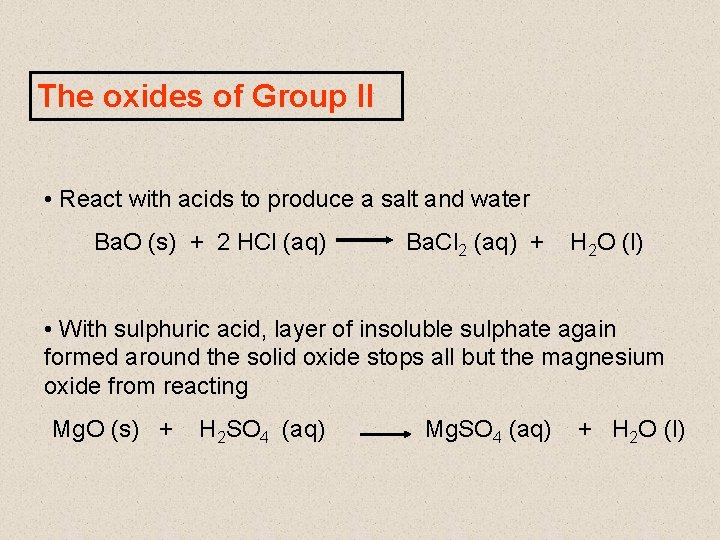

- Slides: 15

The Elements of Group II The Alkaline Earth Metals

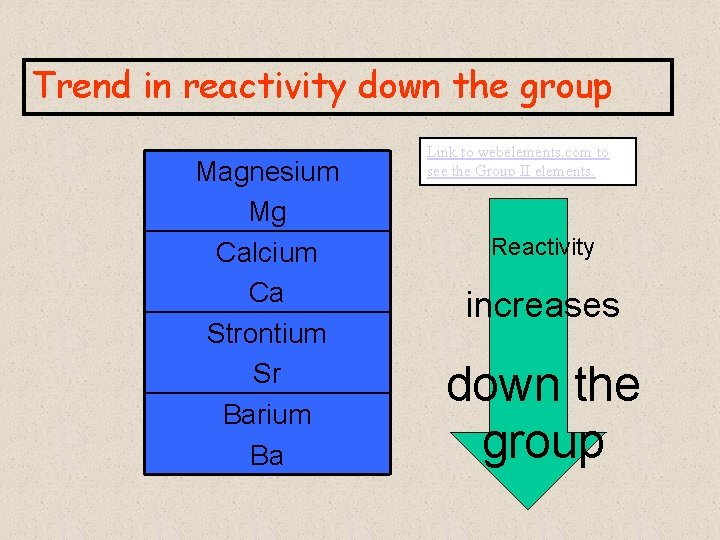
Trend in reactivity down the group Magnesium Mg Calcium Ca Strontium Sr Barium Ba Link to webelements. com to see the Group II elements. Reactivity increases down the group

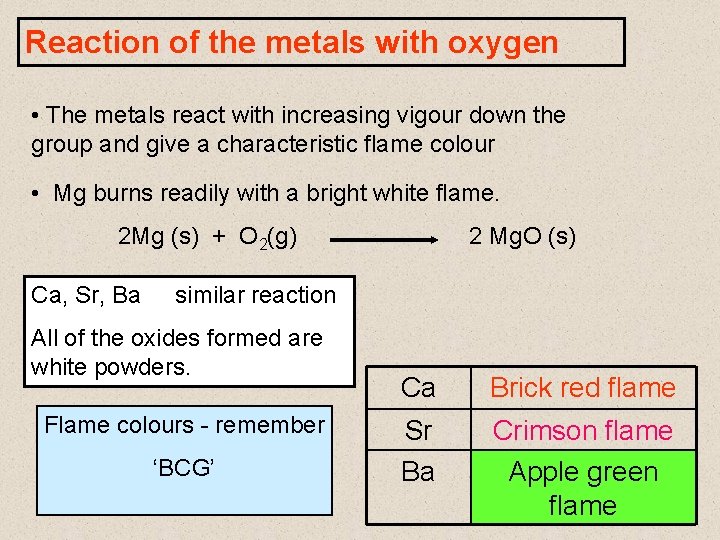
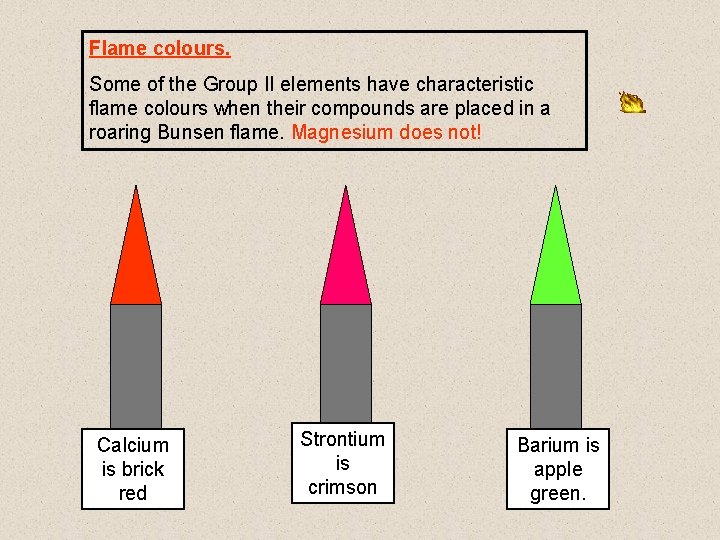
Reaction of the metals with oxygen • The metals react with increasing vigour down the group and give a characteristic flame colour • Mg burns readily with a bright white flame. 2 Mg (s) + O 2(g) Ca, Sr, Ba 2 Mg. O (s) similar reaction All of the oxides formed are white powders. Flame colours - remember ‘BCG’ Ca Brick red flame Sr Ba Crimson flame Apple green flame

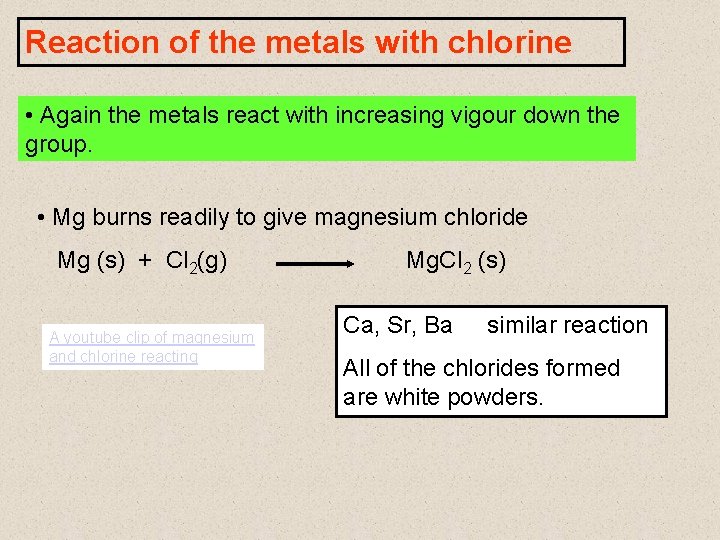
Reaction of the metals with chlorine • Again the metals react with increasing vigour down the group. • Mg burns readily to give magnesium chloride Mg (s) + Cl 2(g) A youtube clip of magnesium and chlorine reacting Mg. Cl 2 (s) Ca, Sr, Ba similar reaction All of the chlorides formed are white powders.

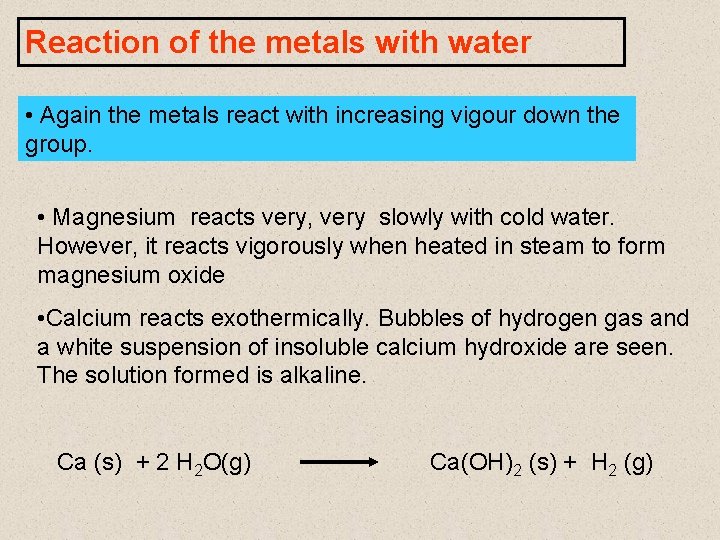
Reaction of the metals with water • Again the metals react with increasing vigour down the group. • Magnesium reacts very, very slowly with cold water. However, it reacts vigorously when heated in steam to form magnesium oxide • Calcium reacts exothermically. Bubbles of hydrogen gas and a white suspension of insoluble calcium hydroxide are seen. The solution formed is alkaline. Ca (s) + 2 H 2 O(g) Ca(OH)2 (s) + H 2 (g)

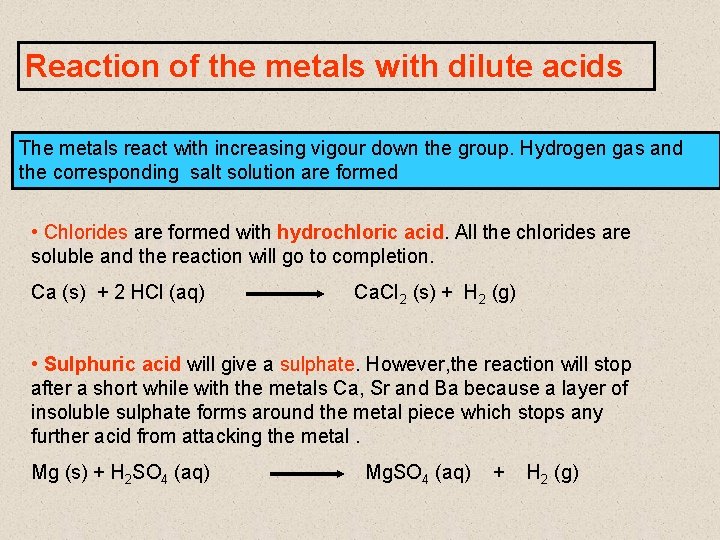
Reaction of the metals with dilute acids The metals react with increasing vigour down the group. Hydrogen gas and the corresponding salt solution are formed • Chlorides are formed with hydrochloric acid. All the chlorides are soluble and the reaction will go to completion. Ca (s) + 2 HCl (aq) Ca. Cl 2 (s) + H 2 (g) • Sulphuric acid will give a sulphate. However, the reaction will stop after a short while with the metals Ca, Sr and Ba because a layer of insoluble sulphate forms around the metal piece which stops any further acid from attacking the metal. Mg (s) + H 2 SO 4 (aq) Mg. SO 4 (aq) + H 2 (g)

Reactions of the aqueous cations with aqueous hydroxide, carbonate and sulphate ions

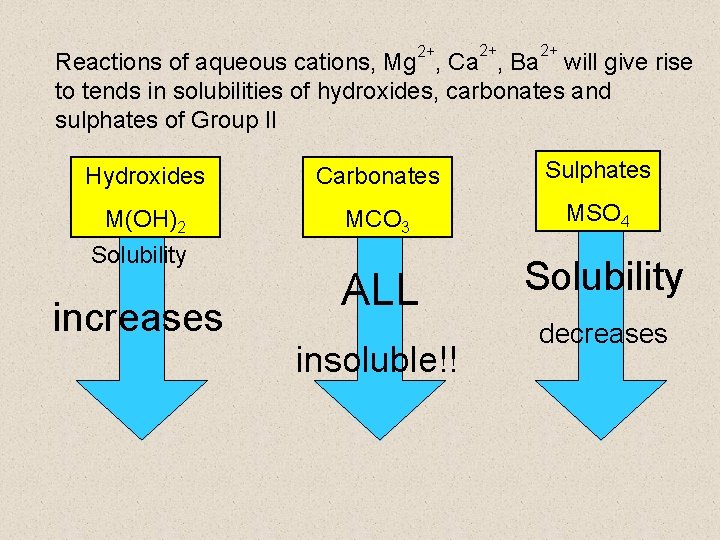
2+ 2+ 2+ Reactions of aqueous cations, Mg , Ca , Ba will give rise to tends in solubilities of hydroxides, carbonates and sulphates of Group II Hydroxides Carbonates Sulphates M(OH)2 MCO 3 MSO 4 ALL Solubility increases insoluble!! decreases

The oxides of Group II • General formula = MO • White, ionic solids with high melting points • All basic oxides • React with water to form the hydroxide Ca. O (s) + H 2 O (l) Ca(OH)2 (aq) • However, Mg. O is insoluble and does not react with water
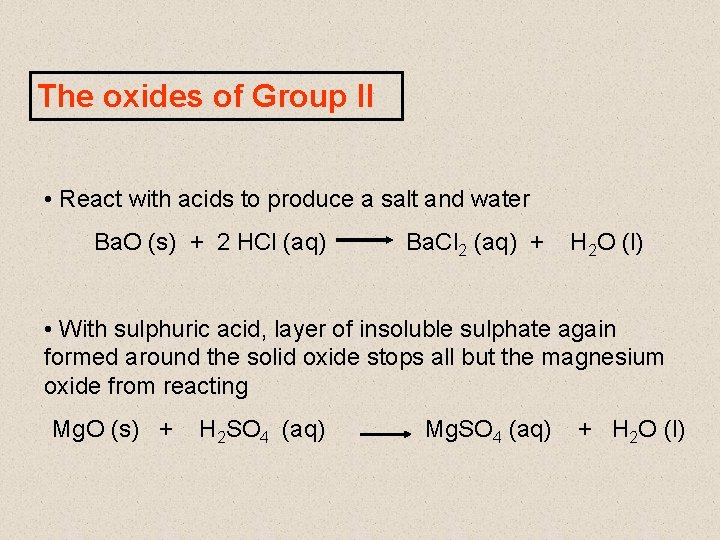
The oxides of Group II • React with acids to produce a salt and water Ba. O (s) + 2 HCl (aq) Ba. Cl 2 (aq) + H 2 O (l) • With sulphuric acid, layer of insoluble sulphate again formed around the solid oxide stops all but the magnesium oxide from reacting Mg. O (s) + H 2 SO 4 (aq) Mg. SO 4 (aq) + H 2 O (l)

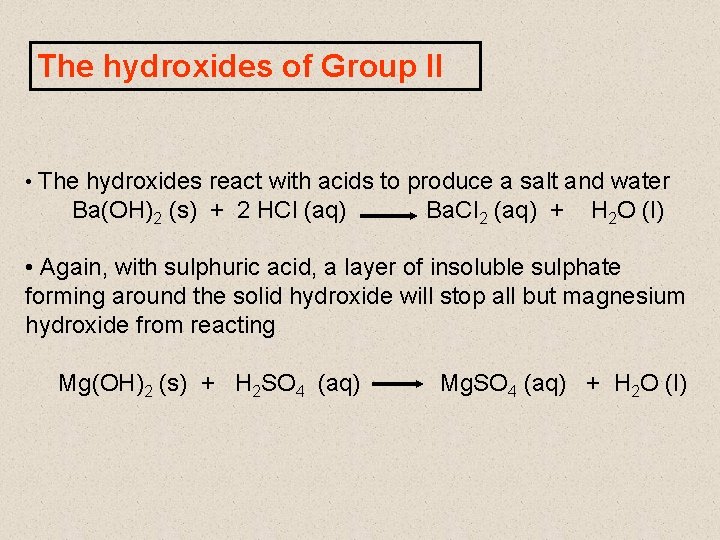
The hydroxides of Group II • General formula = M(OH)2 • White, ionic solids. • All strongly basic hydroxides • Solubility increases as you go down the Group Mg(OH)2 is insoluble Ca(OH)2 is sparingly soluble, and a saturated solution is limewater

The hydroxides of Group II • The hydroxides react with acids to produce a salt and water Ba(OH)2 (s) + 2 HCl (aq) Ba. Cl 2 (aq) + H 2 O (l) • Again, with sulphuric acid, a layer of insoluble sulphate forming around the solid hydroxide will stop all but magnesium hydroxide from reacting Mg(OH)2 (s) + H 2 SO 4 (aq) Mg. SO 4 (aq) + H 2 O (l)

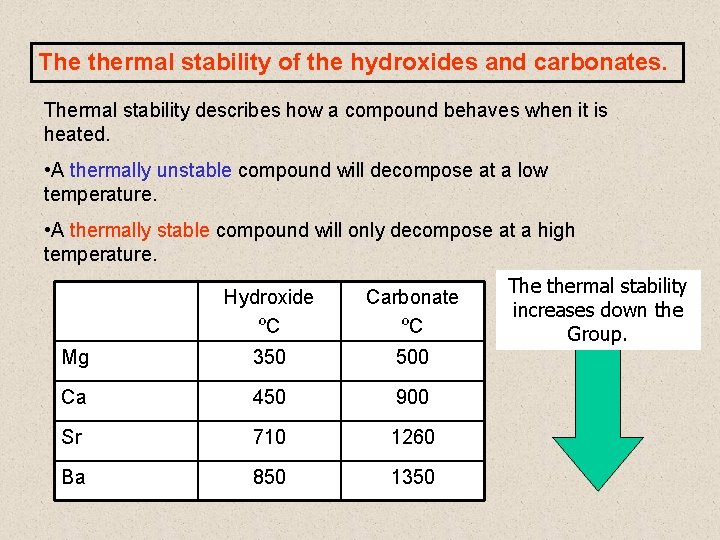
The thermal stability of the hydroxides and carbonates. Thermal stability describes how a compound behaves when it is heated. • A thermally unstable compound will decompose at a low temperature. • A thermally stable compound will only decompose at a high temperature. Hydroxide ºC Carbonate ºC Mg 350 500 Ca 450 900 Sr 710 1260 Ba 850 1350 The thermal stability increases down the Group.

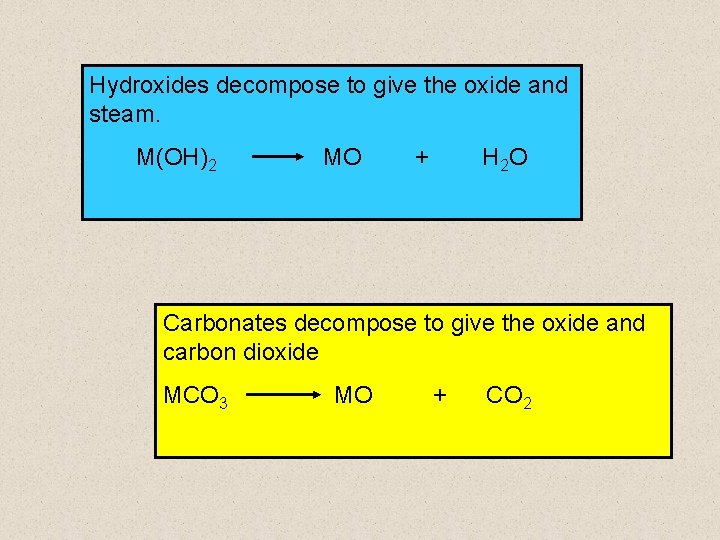
Hydroxides decompose to give the oxide and steam. M(OH)2 MO + H 2 O Carbonates decompose to give the oxide and carbon dioxide MCO 3 MO + CO 2

Flame colours. Some of the Group II elements have characteristic flame colours when their compounds are placed in a roaring Bunsen flame. Magnesium does not! Calcium is brick red Strontium is crimson Barium is apple green.