The electromagnetic spectrum is all wavelengths of em


![Spectra can show what stars and nebulae are made of. [Of stars] “We may Spectra can show what stars and nebulae are made of. [Of stars] “We may](https://slidetodoc.com/presentation_image_h2/7e148f8819f1fa57e00a01bf17b8e931/image-4.jpg)


- Slides: 14

The electromagnetic spectrum is all wavelengths of e/m radiation, both visible (“light”) and invisible (“radiation”).

In 1665 -7, Isaac Newton used two prisms to show that white light is made of all the colors of light. A spectrum is light dispersed into its colors. And of course: wavelength is color.

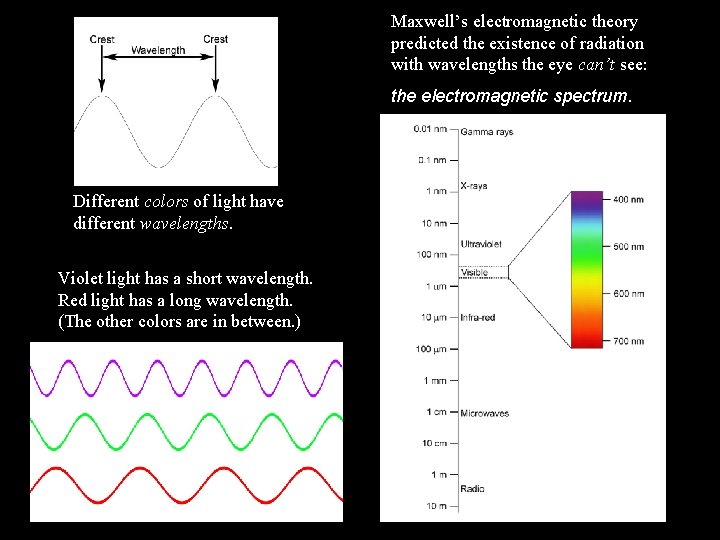
Maxwell’s electromagnetic theory predicted the existence of radiation with wavelengths the eye can’t see: the electromagnetic spectrum. Different colors of light have different wavelengths. Violet light has a short wavelength. Red light has a long wavelength. (The other colors are in between. )
![Spectra can show what stars and nebulae are made of Of stars We may Spectra can show what stars and nebulae are made of. [Of stars] “We may](https://slidetodoc.com/presentation_image_h2/7e148f8819f1fa57e00a01bf17b8e931/image-4.jpg)
Spectra can show what stars and nebulae are made of. [Of stars] “We may determine their forms, their distances, their sizes, and their motions—but we can never know anything of their chemical composition…” – Auguste Comte (philosopher), 1835

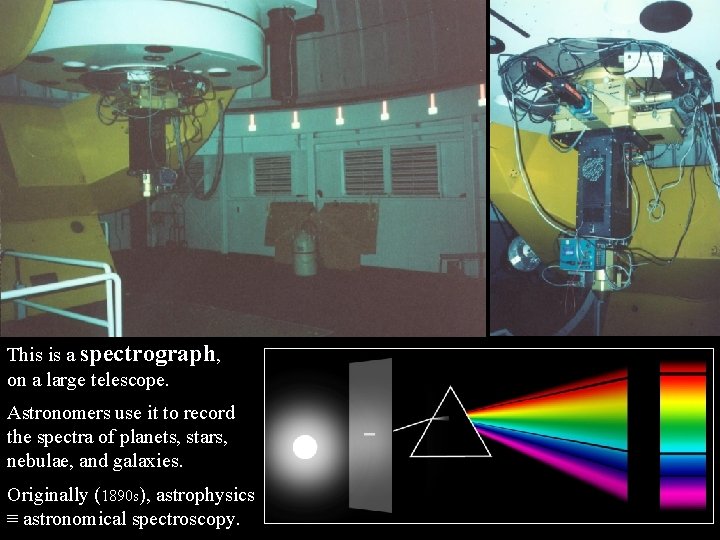
This is a spectrograph, on a large telescope. Astronomers use it to record the spectra of planets, stars, nebulae, and galaxies. Originally (1890 s), astrophysics ≡ astronomical spectroscopy.

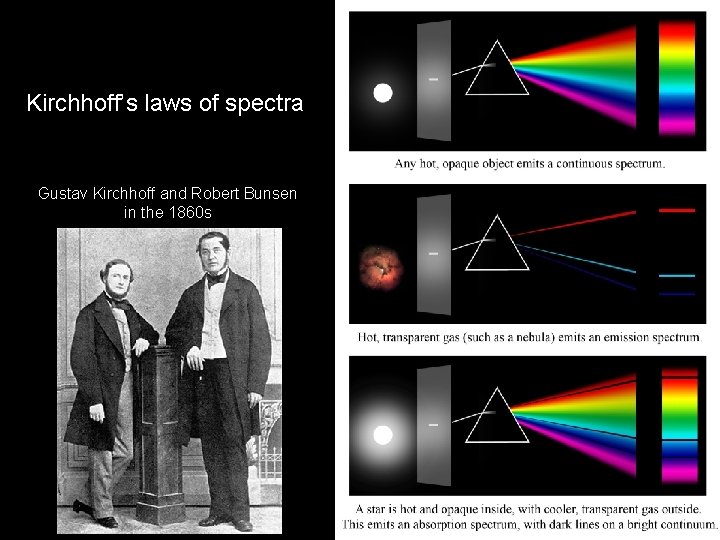
Kirchhoff’s laws of spectra Gustav Kirchhoff and Robert Bunsen in the 1860 s

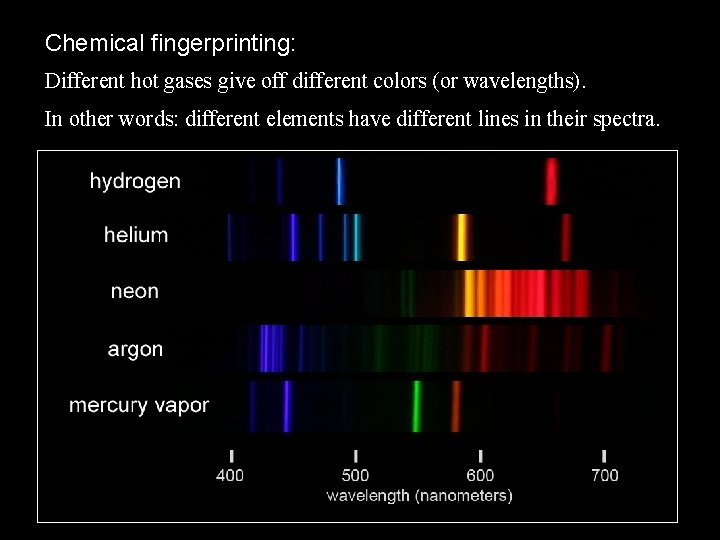
Chemical fingerprinting: Different hot gases give off different colors (or wavelengths). In other words: different elements have different lines in their spectra.

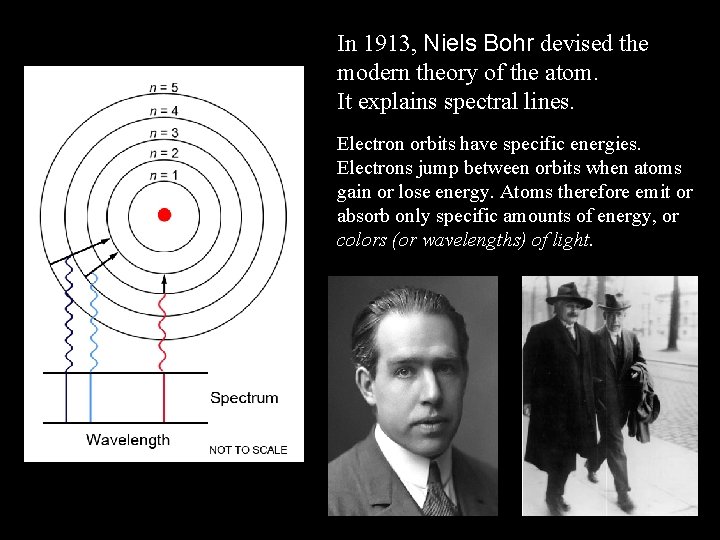
In 1913, Niels Bohr devised the modern theory of the atom. It explains spectral lines. Electron orbits have specific energies. Electrons jump between orbits when atoms gain or lose energy. Atoms therefore emit or absorb only specific amounts of energy, or colors (or wavelengths) of light.



Radiative processes are how stars etc. make light. Most visible light in the Universe follows Kirchhoff’s laws, because it is either: (1) Thermal radiation, from opaque (non-transparent) objects such as stars and planets. Thermal radiation has a continuous spectrum (spread out over many wavelengths): it is therefore said to be a type of continuum radiation. Thermal radiation is the most common kind of e/m radiation in the Universe, both at visible wavelengths (from stars) and at all wavelengths (in the cosmic microwave background radiation). (2) Line radiation, from transparent objects such as nebulae (gas clouds). Line radiation is emitted only at specific wavelengths (colors). Much e/m radiation in the Universe that the unaided eye can’t see (such as X-rays or radio waves) is made by other physical processes, called non-thermal radiative processes. This includes different types of continuum radiation (spread out over a broad range of wavelengths, called a continuum). These include: • synchrotron radiation, from relativistic electrons in magnetic fields (in X-rays, visible light, and long-wavelength radio), • cyclotron radiation, from non-relativistic electrons in magnetic fields, • Bremsstrahlung, “braking radiation” from electrons coming to a sudden stop (in X-rays). Non-thermal types of line radiation (only at specific wavelengths) include: • matter/antimatter annihilation (in gamma rays), • maser radiation, coherent microwave radiation, • laser radiation, coherent visible light, and for completeness: • narrow-band radio signals of intelligent origin (hypothesized: not yet discovered).

Thermal radiation is the light that any hot, opaque object radiates, because it’s hot. (This is sometimes called blackbody radiation, since an object at T = 0 K would be black. It’s sometimes called Planck radiation, since Max Planck figured out its spectrum. ) Examples include a red-hot poker in a fire, the fire itself, the Sun and the stars.

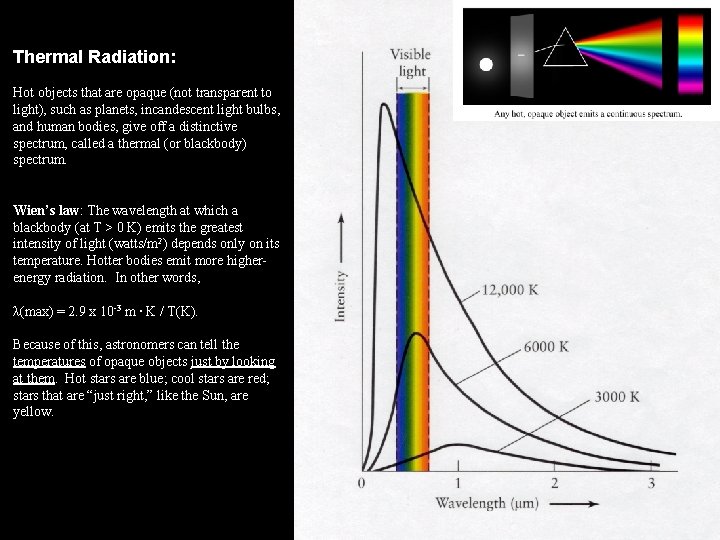
Thermal Radiation: Hot objects that are opaque (not transparent to light), such as planets, incandescent light bulbs, and human bodies, give off a distinctive spectrum, called a thermal (or blackbody) spectrum. Wien’s law: The wavelength at which a blackbody (at T > 0 K) emits the greatest intensity of light (watts/m 2) depends only on its temperature. Hotter bodies emit more higherenergy radiation. In other words, λ(max) = 2. 9 x 10 -3 m ∙ K / T(K). Because of this, astronomers can tell the temperatures of opaque objects just by looking at them. Hot stars are blue; cool stars are red; stars that are “just right, ” like the Sun, are yellow.

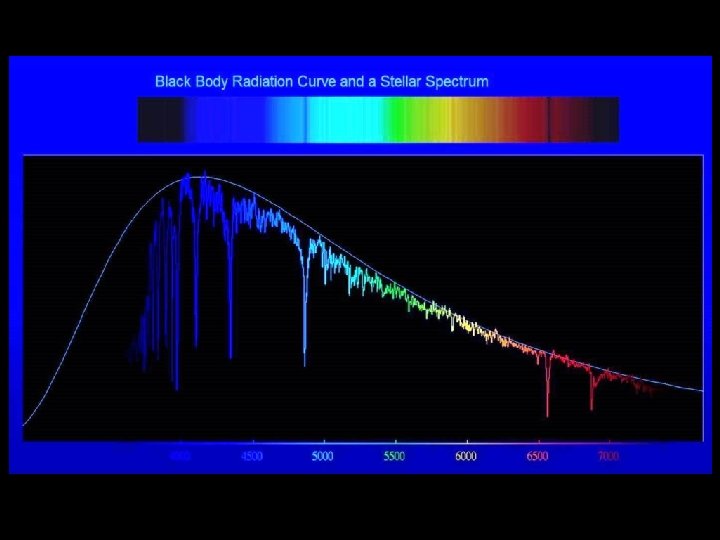
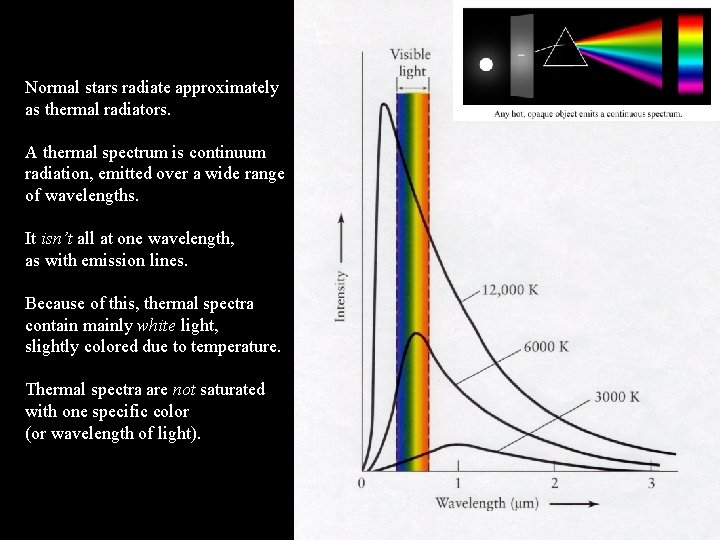
Normal stars radiate approximately as thermal radiators. A thermal spectrum is continuum radiation, emitted over a wide range of wavelengths. It isn’t all at one wavelength, as with emission lines. Because of this, thermal spectra contain mainly white light, slightly colored due to temperature. Thermal spectra are not saturated with one specific color (or wavelength of light).