The EASI recommendations for standardization of ANA tests
























- Slides: 27

The EASI - recommendations for standardization of ANA tests Joined by the IUIS Dr. Nancy Agmon-Levin The Zabludowicz center for Autoimmune diseases, Sheba medical center, Israel

THE PROBLEM OF AUTOANTIBODIES REPRODUCIBILITY IS BURNING !! ACR TASK FORCE - Meroni PL

European countries EASI Israel, Italy, Germany, Netherlands, France, United Kingdom, Sweden, Belgium, Finland, Switzerland, Austria, Spain and Portugal.

The aims of EASI - ANA standardization initiative • To create a set of recommendations for standardized detection of the “ANA family autoantibodies”: • ANA • Anti-ds. DNA antibodies • Specific antigens (“ENA”) • This will enable each country to create a local / adopted algorithm according to local needs and regulations.

EASI - recommendations for ANA • • A list of 31 recommendations (for determination of ANA, anti-ds. DNA and “ENA”) was issued by the EASI committee based on 4 algorithms from Italy, Germany, Netherlands and the ACR as well as personal knowledge. Each recommendation was graded by each EASI team accordingly: (1) Full agreement (2) Major agreement (3) Partial agreement (4) Little agreement (5) No agreement • In addition – comments and suggestions were added For instance: the Israeli score comprise of the average grades given by 17 physician , Ph. D and Lab. technicians. • Final score for each statements = the average of 13 European grades • This was converted into a final set of recommendations • For which we were joined by our colleagues from the IUIS-WHO-CDC •

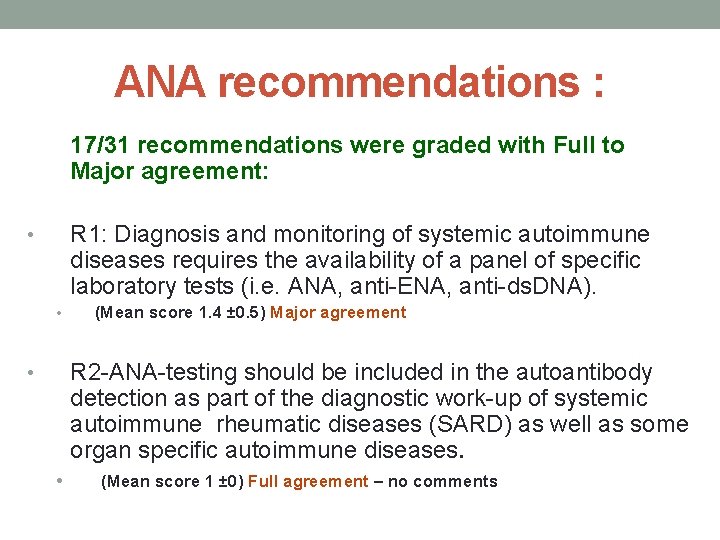
ANA recommendations : 17/31 recommendations were graded with Full to Major agreement: R 1: Diagnosis and monitoring of systemic autoimmune diseases requires the availability of a panel of specific laboratory tests (i. e. ANA, anti-ENA, anti-ds. DNA). • • (Mean score 1. 4 ± 0. 5) Major agreement R 2 -ANA-testing should be included in the autoantibody detection as part of the diagnostic work-up of systemic autoimmune rheumatic diseases (SARD) as well as some organ specific autoimmune diseases. • • (Mean score 1 ± 0) Full agreement – no comments

14 “problematic” recommendations which stands for 5 major issues --- mostly solved 1. 2. 3. 4. 5. ANA (IIFA alternatives and dilutions) The FARR assay alternatives for antids. DNA “ENA” – are repetitive tests required. Should each country/lab calculate its own cut-off levels? Communications between the Lab and the clinicians

The determination of ANA

R 5 - The screening test for ANA: • Immunofluorescence ANA test should remain the gold standard for ANA testing. • (Score 1. 2 ± 0. 4) Major agreement IIF has limitations : The lack of equipment standardization The lack of qualified technicians

The new R 5 recommendation: • Indirect Immunofluorescence (IIF) assay is the standard method for ANA screening. Alternative assays (i. e. ELISA, addressable laser bead immunoassays (ALBIA), chemiluminescent and others) can be used while keeping in mind that false negative and false positive ratio’s of these methods may be higher. Thus, if the clinical suspicion is strong and the alternative method is negative, it is mandatory to perform IIF assay. • Comments : this stands in agreement with the ACR a note regarding the urgent need for training of qualified personnel will be added to text

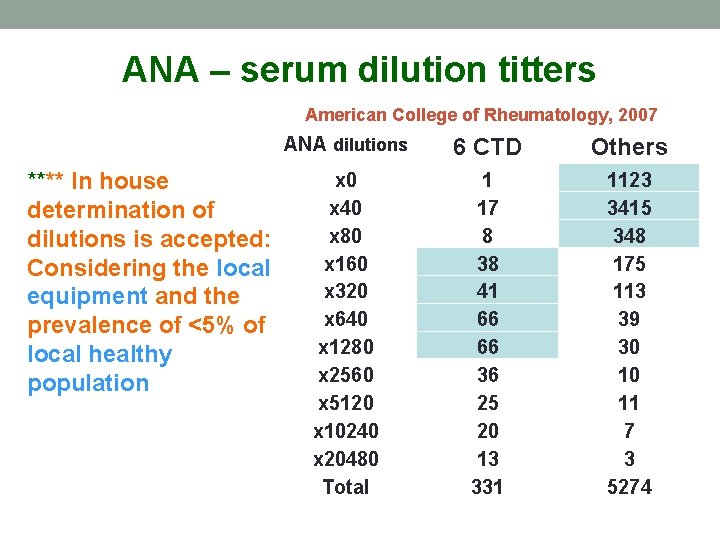
R 10 – The serum dilution for ANA screening • A serum dilution of 1: 80 is to be used in screening procedures. • (average score 2. 9 ± 1. 3) Partial to little agreement

ANA – serum dilution titters American College of Rheumatology, 2007 **** In house determination of dilutions is accepted: Considering the local equipment and the prevalence of <5% of local healthy population ANA dilutions 6 CTD Others x 0 x 40 x 80 x 160 x 320 x 640 x 1280 x 2560 x 5120 x 10240 x 20480 Total 1 17 8 38 41 66 66 36 25 20 13 331 1123 3415 348 175 113 39 30 10 11 7 3 5274

The new R 10 recommendation: A proper IIF assay ANA screening dilution should be defined locally, dependent on reagents, equipment and other local factors. An abnormal ANA should be the titer above the 95 th percentile of a healthy control population. A screening dilution of 1/160 is often suitable for the detection of ANA in adult populations being evaluated for SARD.

The determination of anti-ds. DNA

Major Problems regarding 3/6 original recommendations regarding anti-ds. DNA antibodies: • • • The Farr-assay is the elected method for SLE diagnosis; IIF testing on Crithidia luciliae is an alternative technique at an initial dilution of 1: 10 (Ig. G isotype); ELISA is not recommended. Monitoring of disease by quantitative determination of anti-ds. DNA antibodies is recommended, using the Farr-assay or, as a second choice, an ELISA, FEIA or Luminex can be used. ELISA is not recommended for detection of antids. DNA antibodies for SLE diagnosis, unless a positive result is confirmed by Farr-assay or IIF testing • (Score 2. 9 ± 1. 2) Partial to little agreement

Major comments regarding the FARR assay: • The Farr-assay historically has been considered as golden • • • standard, but this may not be valid anymore. The statement is not in line with the data used for the evidence based guidelines of the ACR (A&R 47, 546 -555, 2002). **ELISA depends on the performance of the ELISA (different assays may significantly differ between each other). **The Farr-assay is the best method but some ELISA are rather adequate and they can be used as first intention testing. The difficulties related to the use of radionuclides are numerous. The Farr-assay is barely used in European countries (in Switzerland the Farr-assay is no longer performed). ELISA is an acceptable alternative as long as its limitations (i. e. false positives) are acknowledged. Crithidia sensitivity can be on the low side.

The new R 15 recommendation • For anti-ds. DNA antibody determination, the Farr assay and the IIF assay on Crithidia luciliae (CLIF) offer higher clinical specificity. Other alternative methods (i. e. ELISA, ALBIA, chemiluminescence etc) yield lower specificity and therefore it is recommended that any positive result by these methods must be confirmed by CLIF Ig. G at 1: 10 or Farr assay.

The determination of ENA

The problematic recommendation regarding the need to repeat “ENA” ` For anti-ENA antibody detection two methods should be used, clearly specifying in the report the methods used and the results obtained with each method. (Score 3. 2 ± 1) Partial to little agreement Impractical and not cost effective The new R 20 recommendation For anti-ENA antibody detection the method used should be reported. An additional technique should be considered in case of discrepancy with IIF assay or with clinical presentation

Should each country/lab calculate its own cut-off levels?

Former recommendation. S (3) for local cutoffs • Each laboratory/country* should calculate its own cut-off level for anti-…. . antibodies by the use of sera from patients with appropiate systemic autoimmune diseases, disease controls and healthy controls, comparable in age and sex; the cut-off should be chosen on the basis of the best compromise between sensitivity and specificity, using ROC curve analysis. • (European score 2. 7 ± 1. 3) Partial to little agreement • Impractical……. . .

The new recommendations: • R 24 - Each country/laboratory should verify the recommended cut-off for kits used to determine ANA. It is recommended to use sera from healthy subjects from the general local population; cut-offs should be defined as the 95 th percentile • R 25. Each country/laboratory should verify the recommended cut-off for kits used to determine anti-ds. DNA and anti-ENA antibodies. It is recommended to use an adequate number of samples from patients with the appropriate autoimmune diseases, disease controls and healthy controls; cut-offs should be defined using ROC curve analysis.

Communication between the Lab and the clinicians

Highly graded recommendations: • R 6 - Laboratories should specify the methods used for detecting ANA when reporting their results. • R 16 - The method used for anti-ds. DNA antibody detection should be communicated to the clinician.

Communication issues to be solved (1): **R 14 - In case of positive ANA results, testing for antids. DNA antibodies is advised when there is clinical suspicion of systemic lupus erythematosus. Should an advice be given to the clinician by the laboratory specialist? Yes - to write a “ laboratory note” (e. g. Homogenous pattern may represent anti-DNA antibodies in SLE patients )

Communication issues to be solved (2): ** R 19 -In case of a positive ANA-test during the diagnostic work-up, depending on pattern, titers and clinical setting, It is recommended to perform specific tests for anti-ENA antibodies. Should the lab decide? YES ! If you can (as part of an algorithm) BUT…. Can not be done in certain countries For these countries/labs maybe to add a “Lab note”? e. g. This pattern may be compatible with…. Or Further studies are recommended according to…. (our reference – we will include tables which specify such data).

Time for comments!