The Earths Carbon Cycle Louisa Bradtmiller Carbon Reservoirs

The Earth’s Carbon Cycle Louisa Bradtmiller
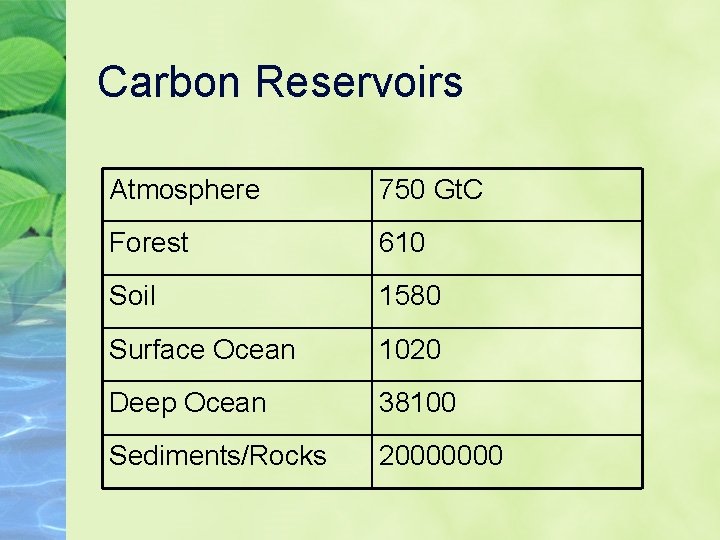
Carbon Reservoirs Atmosphere 750 Gt. C Forest 610 Soil 1580 Surface Ocean 1020 Deep Ocean 38100 Sediments/Rocks 20000000

Size matters How much carbon a reservoir can exchange with other reservoirs depends on Size Rate of exchange So, if we’re interested in atmospheric CO 2, there a few things to keep in mind

Size matters Seems like sediments would be the most likely regulator of atm. CO 2, right? But, they recycle verrrrry slooooowly On tectonic timescales, though, they can control climate to some extent Increased volcanism, seafloor spreading 100 Ma Increased weathering due to Himalayas

Size matters When our cars/factories emit carbon, it goes into the atmosphere 5. 4 Gt. C/yr from fossil fuel burning 1 Gt. C/yr from deforestation BUT not all of it stays there! Approx. 55% stays in the atm. 30% goes into the ocean, and the rest (15%) goes to “greening” (we think).

Atmosphere We know how much goes into the atmosphere, because we can measure CO 2 and CH 4 levels. We know where it comes from, because each source has a different isotopic signature Fossil fuels are “dead carbon”, -2‰ 13 C Plants average -23‰

Terrestrial Biosphere Plants take up CO 2 during photosynthesis, give it off during respiration (ie. burning, decay) So, as long as they’re alive (or in early stages of decay) they store carbon Plants take up 12 C preferentially, giving them negative 13 C values

Terrestrial Biosphere How can trees take up MORE carbon? “Greening” involves the stimulation of increased photosynthesis via increased CO 2 levels in the atm. Verified in greenhouse experiments Another possibility is that anthropogenic N helps to fertilize plants
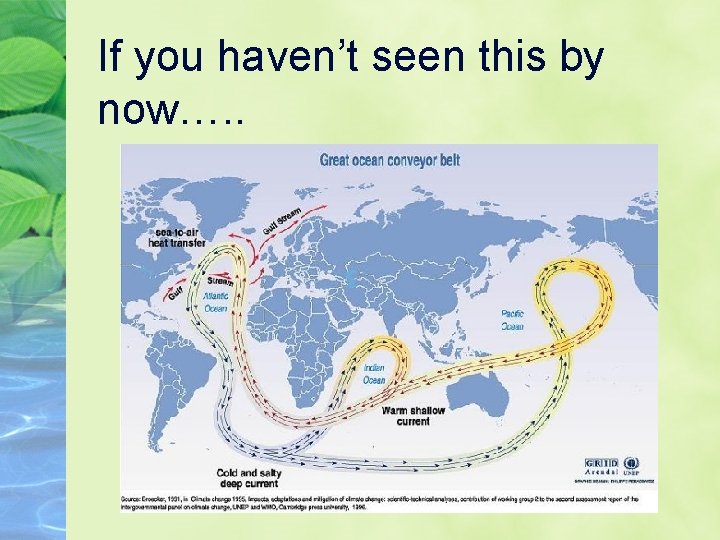
If you haven’t seen this by now…. .

Ocean Carbon Remember, there are 2 reservoirs: Surface (upper 100 meters) Deep (everything else) Only the surface really “communicates” with the atmosphere- they are in equilibrium So, is that where the carbon goes?

Ocean Carbon NOPE. The surface ocean is too small to accommodate that much C, and exchanges too quickly (it would put it right back in the atm. In a few years) So, it must be going into the deep ocean (thus the conveyor picture)

How I learned to stop worrying and love the bomb The deep ocean “overturns” on a timescale of about 1500 years. This is long enough to store some serious C How do we know that, anyway? Bomb 14 C- nuclear tests in the 50’s and 60’s put tons if it into the Atm. Folks (esp. Wally) watched how it was incorporated into the deep ocean

Ocean carbon “pumps” 3 types: Solubility organic, or soft tissue Carbonate, or inorganic Solubility refers to the fact that gases dissolve more easily in cold water So, a colder ocean holds more CO 2
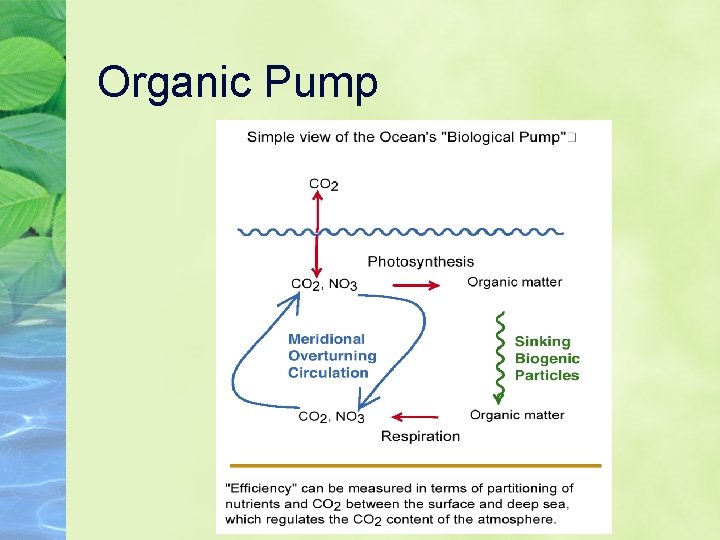
Organic Pump
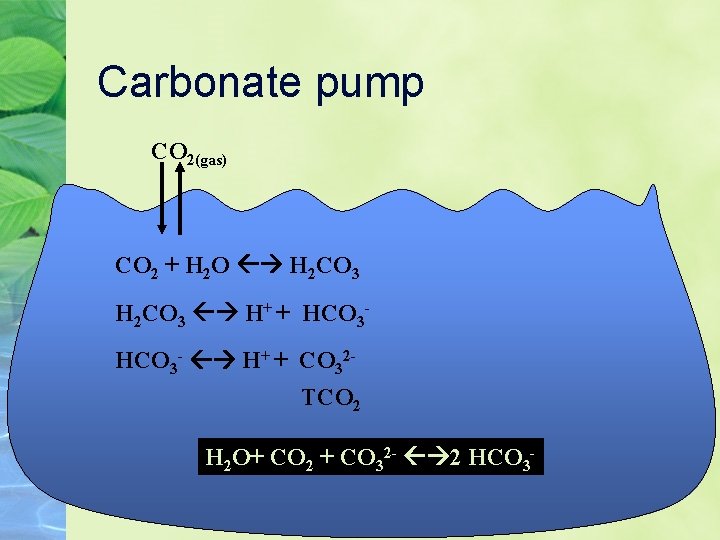
Carbonate pump CO 2(gas) CO 2 + H 2 O H 2 CO 3 H+ + HCO 3 - H+ + CO 32 TCO 2 H 2 O+ CO 2 + CO 32 - 2 HCO 3 -

Carbonate pump In addition, many organisms make Ca. CO 3 shells. The solubility of these shells on the seafloor is determined by the concentration of CO 32 So, shells are preserved in shallow water, but not below the lysocline

Carbonate pump The depth of the lysocline can change with time Easy to remember: CO 2 ~ 1/CO 32 So, during a glacial, there is low CO 2 and you preserve more carbonate Or, the lysocline deepens

Final thoughts The ocean must be the regulator of modern (glacial-interglacial) carbon changes- it’s the only reservoir that is big enough and exchanges on an appropriate timescale
- Slides: 18