The Drinking Water Treatment Process Why do we














- Slides: 18

The Drinking Water Treatment Process

Why do we need to treat water? n There are many impurities in the raw water n These impurities can be grouped into three categories: n Physical: – materials that do not dissolve in water and make the water appear "dirty" n Chemical: substances dissolved in the water from both natural and man-made processes n Biological: viruses, bacteria, algae, and other small living organisms.

Is the drinking water that comes out of our tap "pure"? n No "Chemically pure" water, entirely free from any other materials, does not exist in nature. n Distilled water, is usually flat and tasteless and few people enjoy drinking it. n It would be prohibitively expensive and possibly unhealthy to purify our entire water supply to that level. n "Natural water", free from any man-made additives (if it still exists) contains concentrations of minerals such as calcium, magnesium, and iron which are beneficial to human health in small quantities.

Drinking water treatment Large particles and debris are removed from the raw water by travelling screens just as the water enters the treatment plants. There are five different processes our water goes through

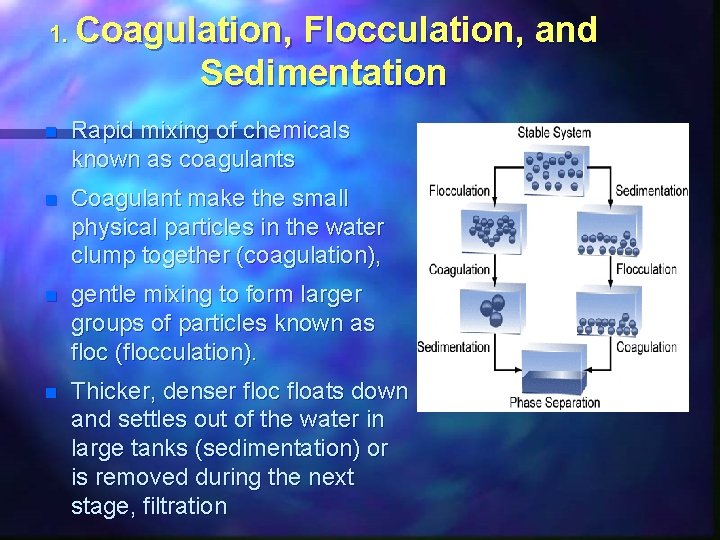
1. Coagulation, Flocculation, and Sedimentation n Rapid mixing of chemicals known as coagulants n Coagulant make the small physical particles in the water clump together (coagulation), n gentle mixing to form larger groups of particles known as floc (flocculation). n Thicker, denser floc floats down and settles out of the water in large tanks (sedimentation) or is removed during the next stage, filtration

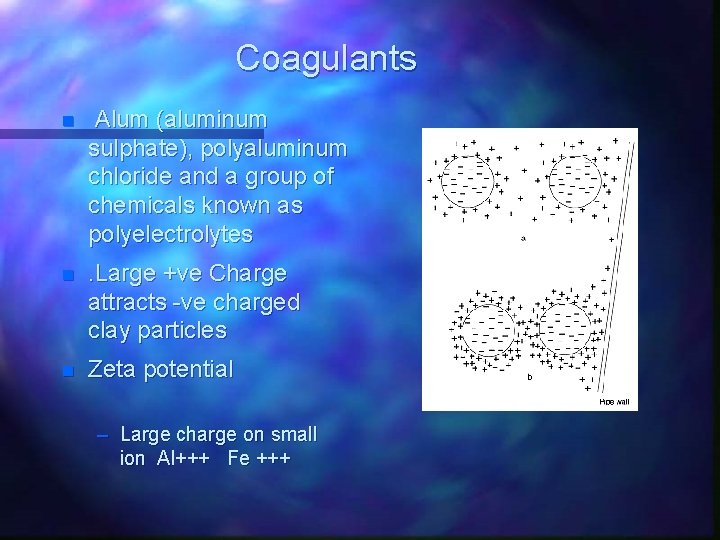
Coagulants n Alum (aluminum sulphate), polyaluminum chloride and a group of chemicals known as polyelectrolytes n . Large +ve Charge attracts -ve charged clay particles n Zeta potential – Large charge on small ion Al+++ Fe +++

Settlement

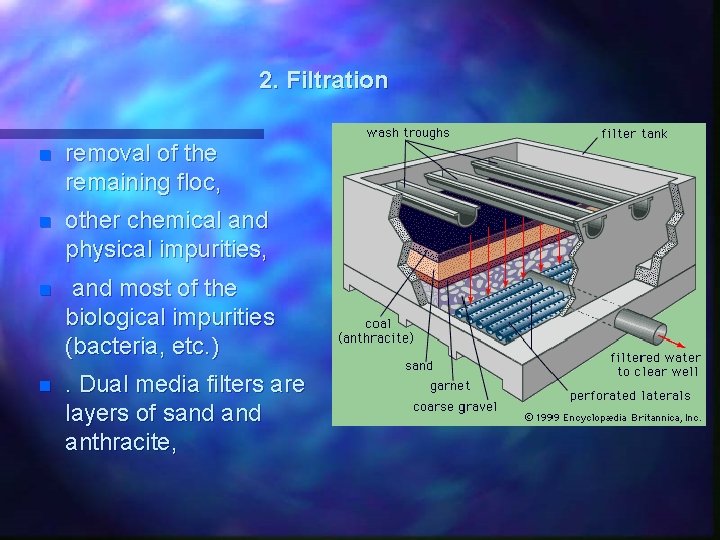
2. Filtration n removal of the remaining floc, n other chemical and physical impurities, n and most of the biological impurities (bacteria, etc. ) n . Dual media filters are layers of sand anthracite,

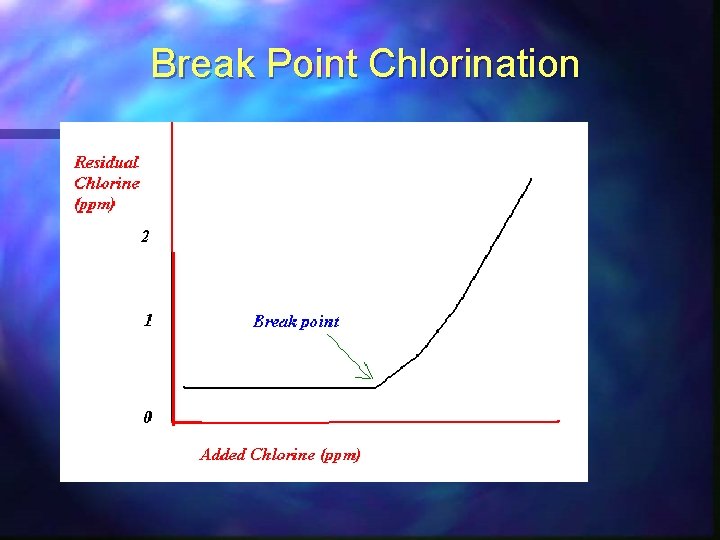
3. Disinfection n The addition of the chemical chlorine, n The chemical is added to our water at different points in the treatment process. n When chlorine is added at beginning prechlorination. n After the filtration stage it is known as postchlorination. n Superchlorination when the levels of bacteria are high. n Sulphur dioxide is then added to remove excess chlorine

Break Point Chlorination

4. Fluoridation n Add additional fluoride after the filtration stage to raise the level to 1. 2 mg/l.

5. Ammoniation n Ammonia is added at the end of the treatment process and combines with the remaining chlorine. n This stabilizes the chlorine so that it remains dissolved in the treated water for longer Ammoniation also prevents chlorine from evaporating out of your drinking water causing smells and associated tastes.

Ground Water Treatment n groundwater is significantly easier to treat than surface water. .

THE TREATMENT PROCESS n Aeration. Raw water pumped from the well is mixed with air. n The mixing releases carbon dioxide and hydrogen sulfide gases present in the water. n Aeration also oxidizes any iron, – causing it to "precipitate" (or settle out) – removed by precipitation and filtration.

Lime (calcium hydroxide) n Added to remove the calcium and magnesium salts. n The p. H of the water is raised from approximately 7. 6 to a range of 10. 4 to 10. 6. n Converts the calcium and magnesium from a soluble to insoluble form, n Ca. CO 3 + Ca (OH)2 --> Ca (HCO 3 )2 + H 20 n causing the insoluble material to precipitate out. n Lime "sludge" on the bottom of the basins , re -use in agriculture.

Recarbonation. n Liquid carbon dioxide is mixed with the water. n The liquid carbon dioxide converts insoluble salts back to soluble salts. n Ca (HCO 3 )2 + CO 2 --> Ca CO 3 + H 20

Filters n Composed of layers of filter sand graded gravel. n These are washed cleaned from the filter bed approximately every 50 hours through a process known as "backwashing". .
