The Distillation and Volatility of Ionic Liquids Martyn

![1 -ethyl-3 -methylimidazolium chloride • [C 2 mim]Cl - Al. Cl 3 system with 1 -ethyl-3 -methylimidazolium chloride • [C 2 mim]Cl - Al. Cl 3 system with](https://slidetodoc.com/presentation_image/ede4368876d598b2847d01ed31df2088/image-2.jpg)
![[NTf 2]- is bis{(trifluoromethyl)-sulphonyl}amide, also known as bistriflamide, [N(SO 2 CF 3)2]- 5 [NTf 2]- is bis{(trifluoromethyl)-sulphonyl}amide, also known as bistriflamide, [N(SO 2 CF 3)2]- 5](https://slidetodoc.com/presentation_image/ede4368876d598b2847d01ed31df2088/image-5.jpg)
![cholinium tetraalkylammonium tetraalkylphosphonium [Cx y z. N(C 2 H 4 OH)]+ [NCw x y cholinium tetraalkylammonium tetraalkylphosphonium [Cx y z. N(C 2 H 4 OH)]+ [NCw x y](https://slidetodoc.com/presentation_image/ede4368876d598b2847d01ed31df2088/image-7.jpg)
![cholinium tetraalkylammonium tetraalkylphosphonium [Cx y z. N(C 2 H 4 OH)]+ [NCw x y cholinium tetraalkylammonium tetraalkylphosphonium [Cx y z. N(C 2 H 4 OH)]+ [NCw x y](https://slidetodoc.com/presentation_image/ede4368876d598b2847d01ed31df2088/image-8.jpg)




- Slides: 14

The Distillation and Volatility of Ionic Liquids Martyn J. Earle, Jose M. S. S. Esperanca, Manuela A. Gilea, Jose N. Canongia Lopes, Luis P. N. Rebelo, Joseph W. Magee, Kenneth R. Seddon & Jason A. Widegren Nature 2006, 439, 831 -834. 1
![1 ethyl3 methylimidazolium chloride C 2 mimCl Al Cl 3 system with 1 -ethyl-3 -methylimidazolium chloride • [C 2 mim]Cl - Al. Cl 3 system with](https://slidetodoc.com/presentation_image/ede4368876d598b2847d01ed31df2088/image-2.jpg)
1 -ethyl-3 -methylimidazolium chloride • [C 2 mim]Cl - Al. Cl 3 system with a greater than 2: 1 excess of Al. Cl 3, a detectable vapour pressure of Al 2 Cl 6 was observed above 191 o. C. • [C 2 mim]Cl 2 190 o. C in vacuum decompose (transalkylation): 1 -methylimidazole, 1 -ethylimidazole, chloromethane, chloroethane, ethene and hydrogen chloride recondense: [C 1 mim]Cl - [C 2 eim]Cl Dymek, C. J. Jr, Hussey, C. L. , Wilkes, J. S. & Øye, H. A. in Joint (Sixth) International Symposium on Molten Salts (eds Mamantov, G. et al. ) 93–-104 (The Electrochemical Society, Pennington, New Jersey, 1987). Øye, H. A. & Wahnsiedler, W. E. in The Fourth International Symposium on Molten Salts (eds Blander, M. , Newman, D. S. , Saboungi, M. L. , Mamantov, G. & Johnson, K. ) 58–-73 (The Electrochemical Society, Pennington, New Jersey, 1984). Jeapes, A. J. et al. Process for recycling ionic liquids. World Patent WO 01/15175 (2001). 2

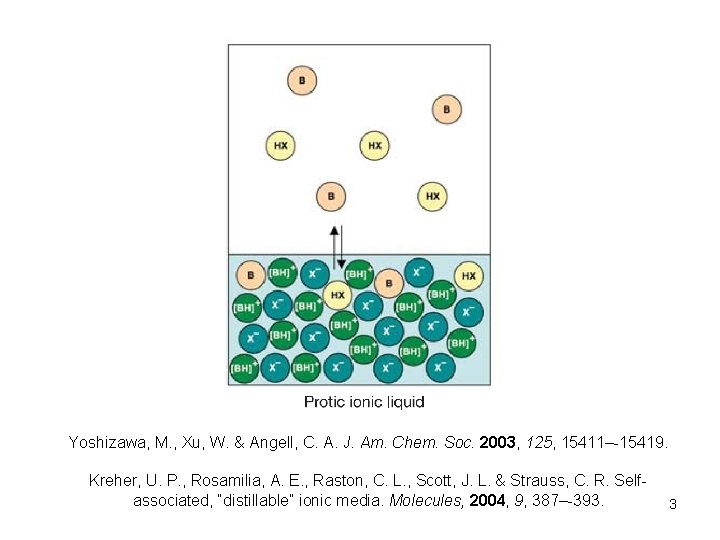
Yoshizawa, M. , Xu, W. & Angell, C. A. J. Am. Chem. Soc. 2003, 125, 15411–-15419. Kreher, U. P. , Rosamilia, A. E. , Raston, C. L. , Scott, J. L. & Strauss, C. R. Selfassociated, “distillable” ionic media. Molecules, 2004, 9, 387–-393. 3

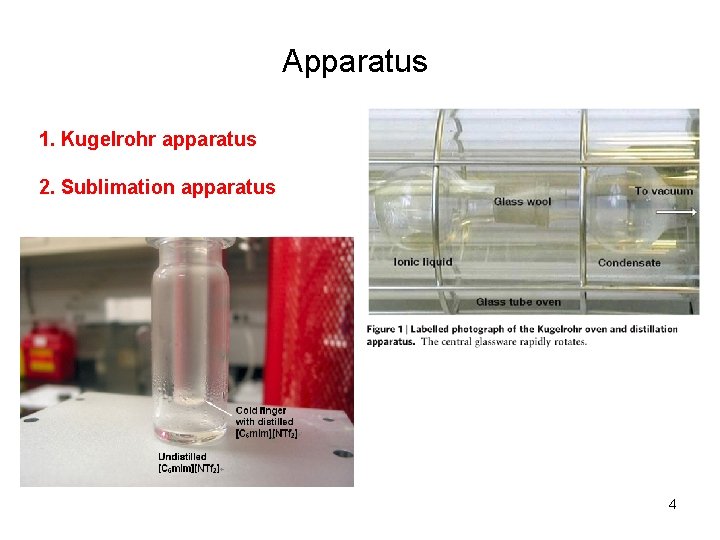
Apparatus 1. Kugelrohr apparatus 2. Sublimation apparatus 4
![NTf 2 is bistrifluoromethylsulphonylamide also known as bistriflamide NSO 2 CF 32 5 [NTf 2]- is bis{(trifluoromethyl)-sulphonyl}amide, also known as bistriflamide, [N(SO 2 CF 3)2]- 5](https://slidetodoc.com/presentation_image/ede4368876d598b2847d01ed31df2088/image-5.jpg)
[NTf 2]- is bis{(trifluoromethyl)-sulphonyl}amide, also known as bistriflamide, [N(SO 2 CF 3)2]- 5

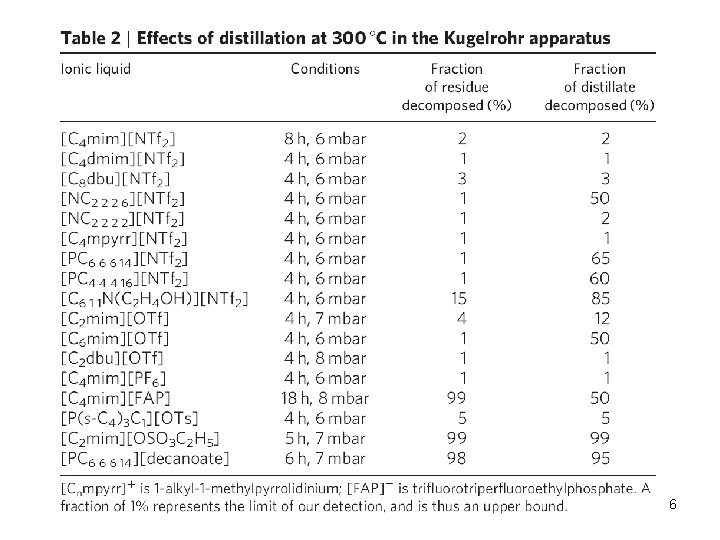
6
![cholinium tetraalkylammonium tetraalkylphosphonium Cx y z NC 2 H 4 OH NCw x y cholinium tetraalkylammonium tetraalkylphosphonium [Cx y z. N(C 2 H 4 OH)]+ [NCw x y](https://slidetodoc.com/presentation_image/ede4368876d598b2847d01ed31df2088/image-7.jpg)
cholinium tetraalkylammonium tetraalkylphosphonium [Cx y z. N(C 2 H 4 OH)]+ [NCw x y z]+ [PCw x y z ]+ 7
![cholinium tetraalkylammonium tetraalkylphosphonium Cx y z NC 2 H 4 OH NCw x y cholinium tetraalkylammonium tetraalkylphosphonium [Cx y z. N(C 2 H 4 OH)]+ [NCw x y](https://slidetodoc.com/presentation_image/ede4368876d598b2847d01ed31df2088/image-8.jpg)
cholinium tetraalkylammonium tetraalkylphosphonium [Cx y z. N(C 2 H 4 OH)]+ [NCw x y z]+ [PCw x y z ]+ ˇ 8

• Ionic liquids with other anions (for example, halides, sulphates, or carboxylates) decomposed on distillation. • The principal mechanism of decomposition was by dealkylation or transalkylation of the cation. • This is aided by a nucleophilic anion. 9

10

11

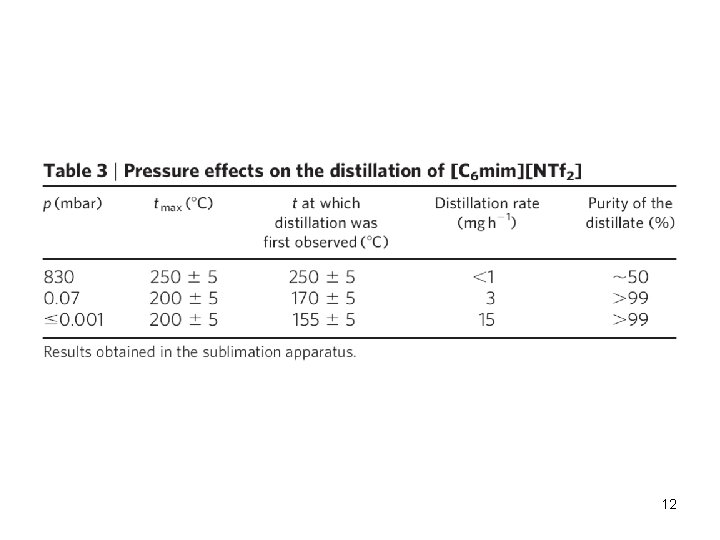
12

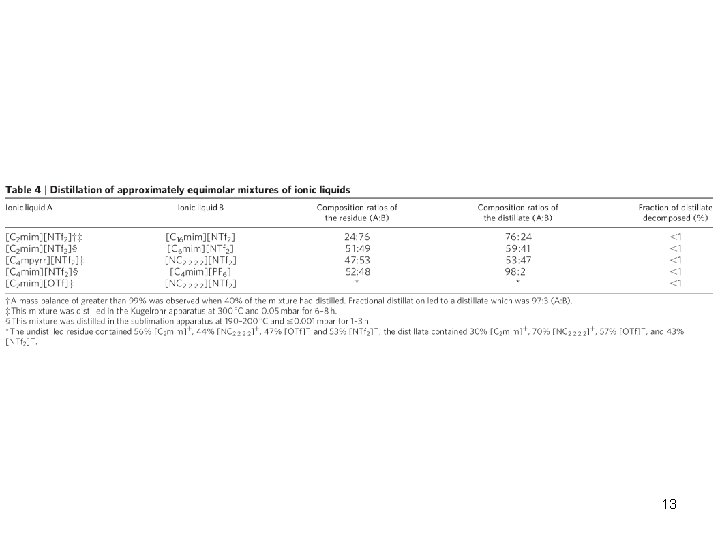
13

No feasible mechanism to transfer a proton to the anion R = H , CH 3 14
 Volatility of ionic compounds
Volatility of ionic compounds Simple distillation boiling point difference
Simple distillation boiling point difference Introduction of distillation
Introduction of distillation Vacuum distillation definition
Vacuum distillation definition Ionic liquids ppt
Ionic liquids ppt ионные уравнения
ионные уравнения Types of distillation
Types of distillation Wceaux.dll
Wceaux.dll How to calculate relative volatility
How to calculate relative volatility Volatility skew
Volatility skew Volatility smile reason
Volatility smile reason Swaption volatility surface
Swaption volatility surface Volatility adjustment
Volatility adjustment 0000ar index
0000ar index Ethanol azeotrope
Ethanol azeotrope