The Discovery of Benzene v Benzene was discovered

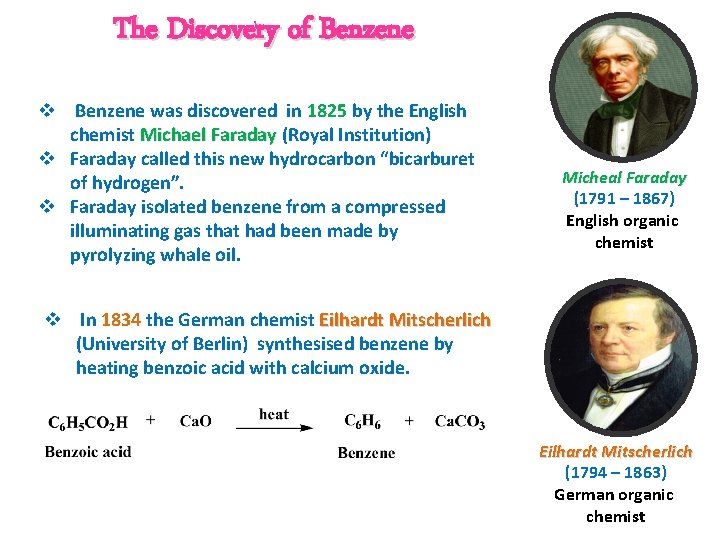
The Discovery of Benzene v Benzene was discovered in 1825 by the English chemist Michael Faraday (Royal Institution) v Faraday called this new hydrocarbon “bicarburet of hydrogen”. v Faraday isolated benzene from a compressed illuminating gas that had been made by pyrolyzing whale oil. Micheal Faraday (1791 – 1867) English organic chemist v In 1834 the German chemist Eilhardt Mitscherlich (University of Berlin) synthesised benzene by heating benzoic acid with calcium oxide. Eilhardt Mitscherlich (1794 – 1863) German organic chemist
Structure of Benzene Friedrich August Kekulé (1829 – 1896) German organic chemist
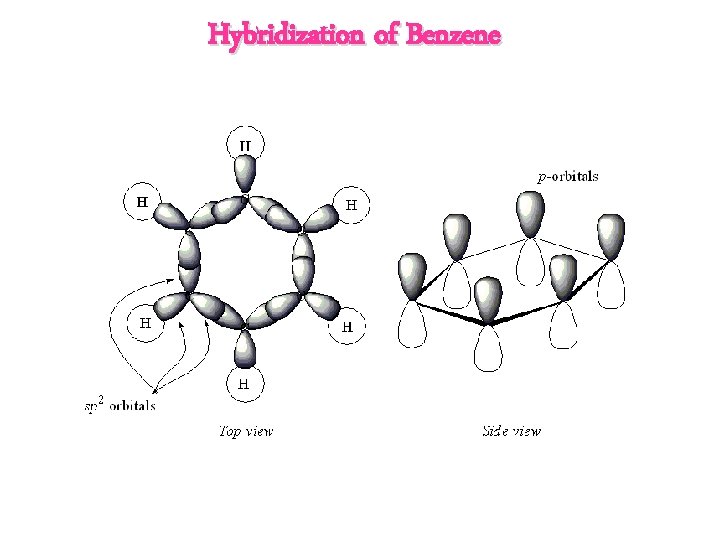
Hybridization of Benzene
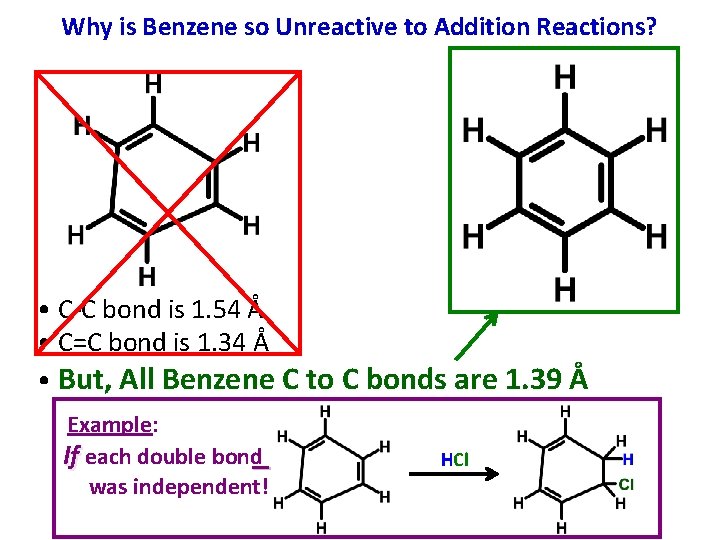
Why is Benzene so Unreactive to Addition Reactions? • C-C bond is 1. 54 Å • C=C bond is 1. 34 Å • But, All Benzene C to C bonds are 1. 39 Å Example: If each double bond was independent! HCl
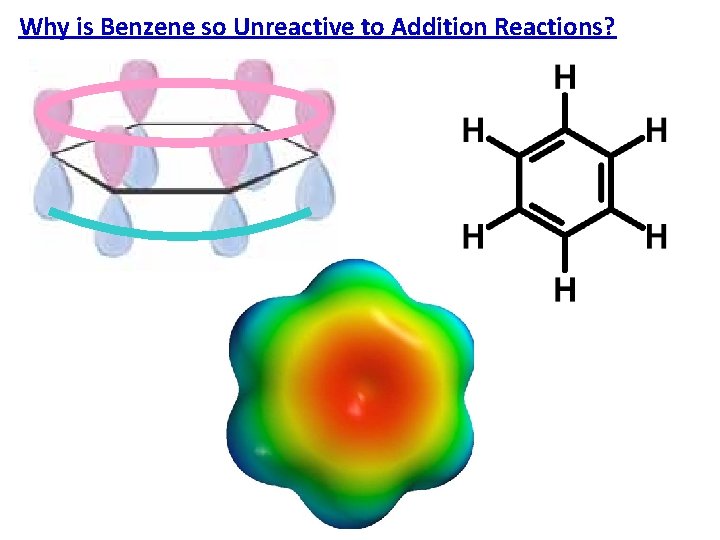
Why is Benzene so Unreactive to Addition Reactions?
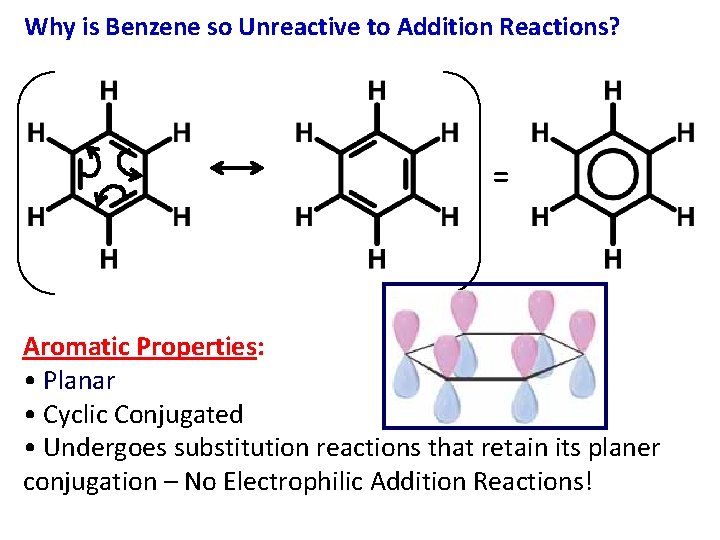
Why is Benzene so Unreactive to Addition Reactions? = Aromatic Properties: • Planar • Cyclic Conjugated • Undergoes substitution reactions that retain its planer conjugation – No Electrophilic Addition Reactions!
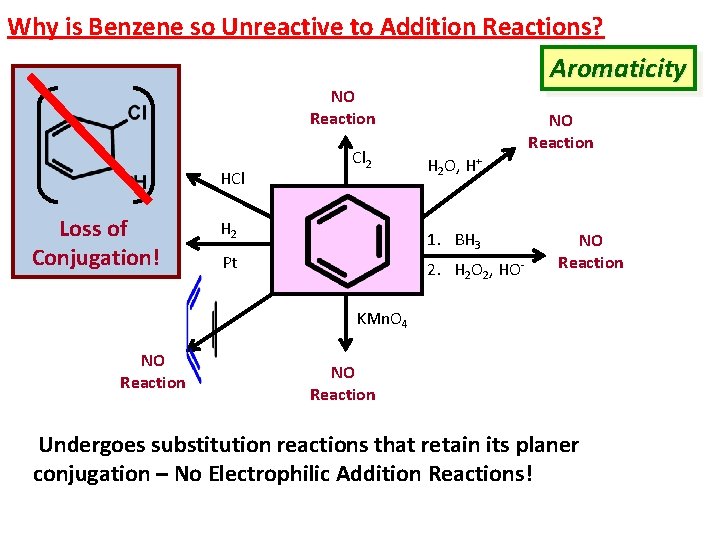
Why is Benzene so Unreactive to Addition Reactions? Aromaticity NO Reaction HCl Loss of NO Reaction Conjugation! Cl 2 H 2 NO Reaction H 2 O, H+ 1. BH 3 Pt 2. H 2 O 2, HO- NO Reaction KMn. O 4 NO Reaction Undergoes substitution reactions that retain its planer conjugation – No Electrophilic Addition Reactions!
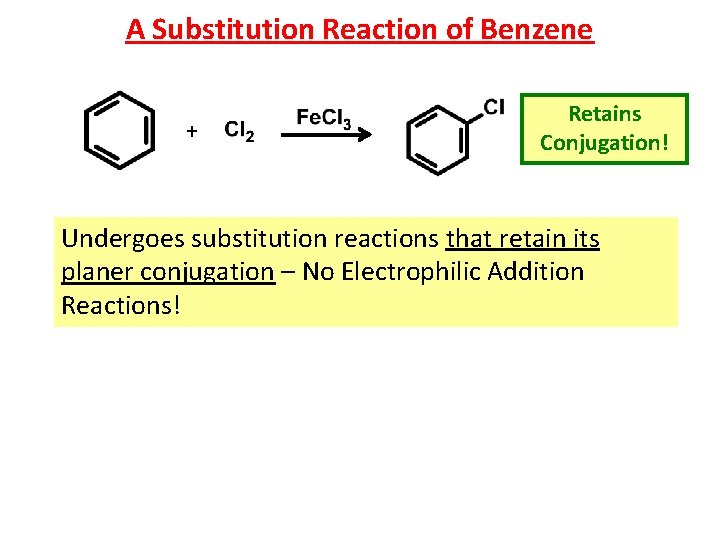
A Substitution Reaction of Benzene + Retains Conjugation! Undergoes substitution reactions that retain its planer conjugation – No Electrophilic Addition Reactions!
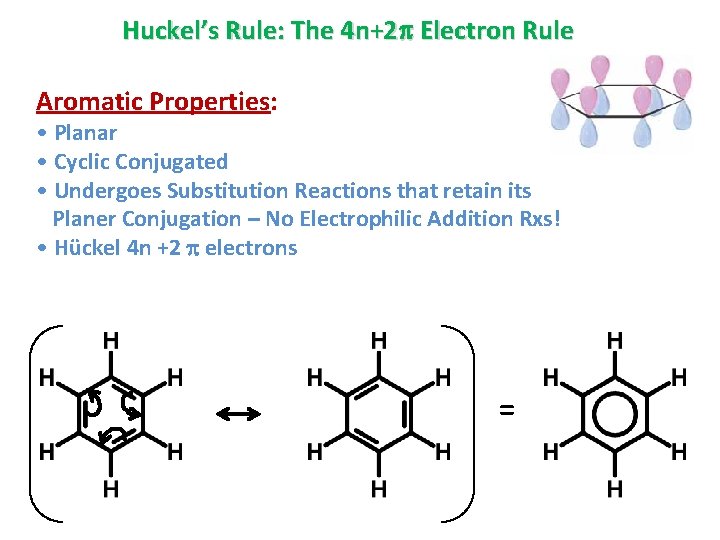
Huckel’s Rule: The 4 n+2 Electron Rule Aromatic Properties: • Planar • Cyclic Conjugated • Undergoes Substitution Reactions that retain its Planer Conjugation – No Electrophilic Addition Rxs! • Hückel 4 n +2 electrons =
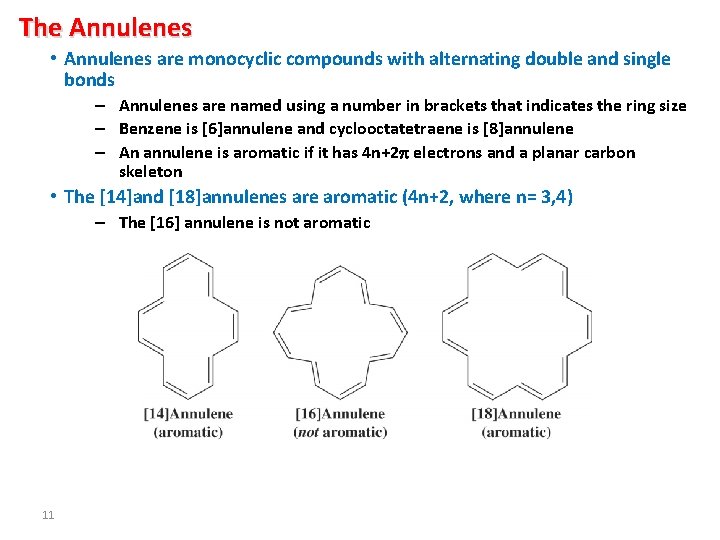
The Annulenes • Annulenes are monocyclic compounds with alternating double and single bonds – Annulenes are named using a number in brackets that indicates the ring size – Benzene is [6]annulene and cyclooctatetraene is [8]annulene – An annulene is aromatic if it has 4 n+2 electrons and a planar carbon skeleton • The [14]and [18]annulenes are aromatic (4 n+2, where n= 3, 4) – The [16] annulene is not aromatic 11
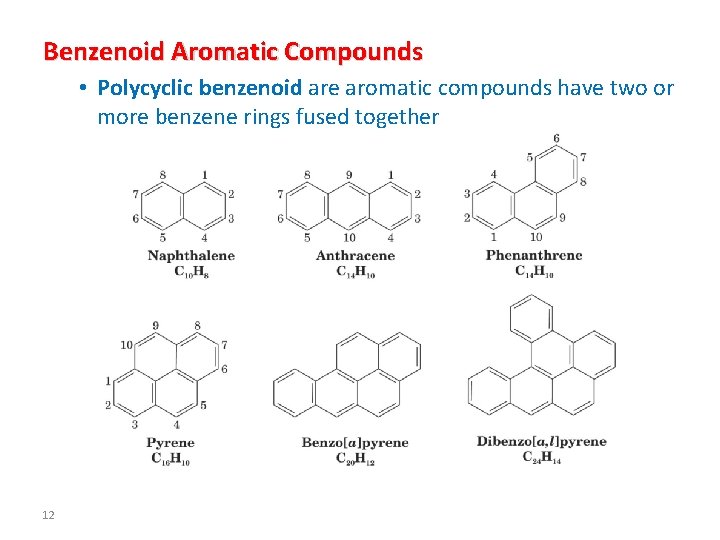
Benzenoid Aromatic Compounds • Polycyclic benzenoid are aromatic compounds have two or more benzene rings fused together 12
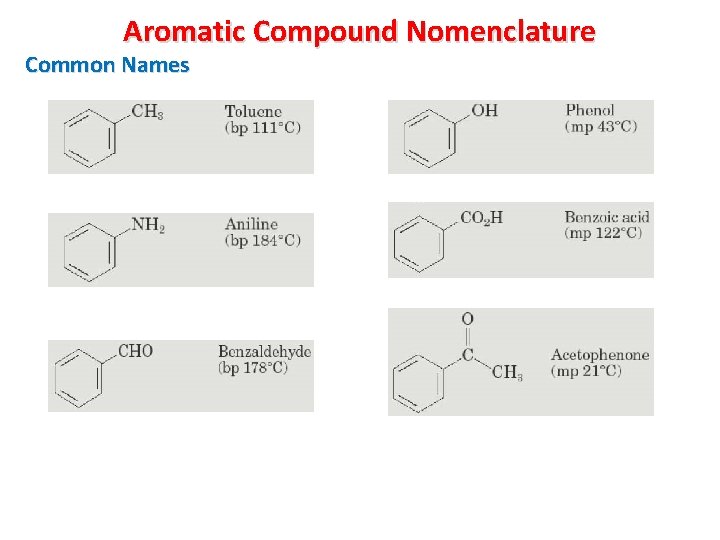
Aromatic Compound Nomenclature Common Names
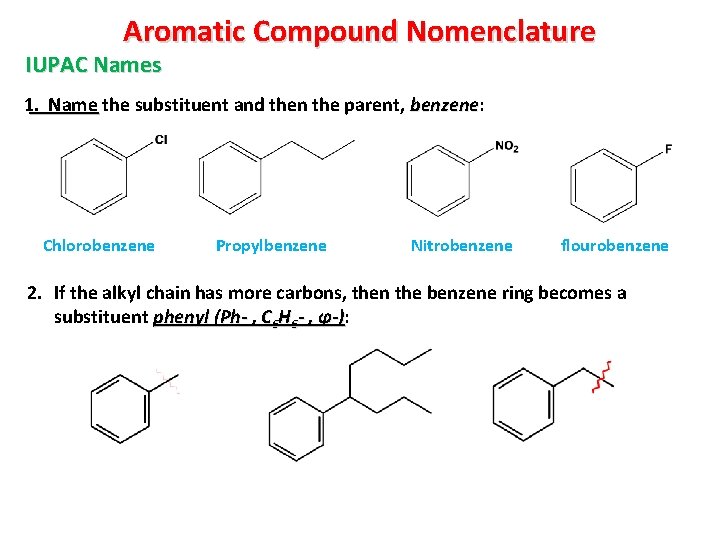
Aromatic Compound Nomenclature IUPAC Names 1. Name the substituent and then the parent, benzene: Chlorobenzene Propylbenzene Nitrobenzene flourobenzene 2. If the alkyl chain has more carbons, then the benzene ring becomes a substituent phenyl (Ph- , C 6 H 6 - , φ-): -)
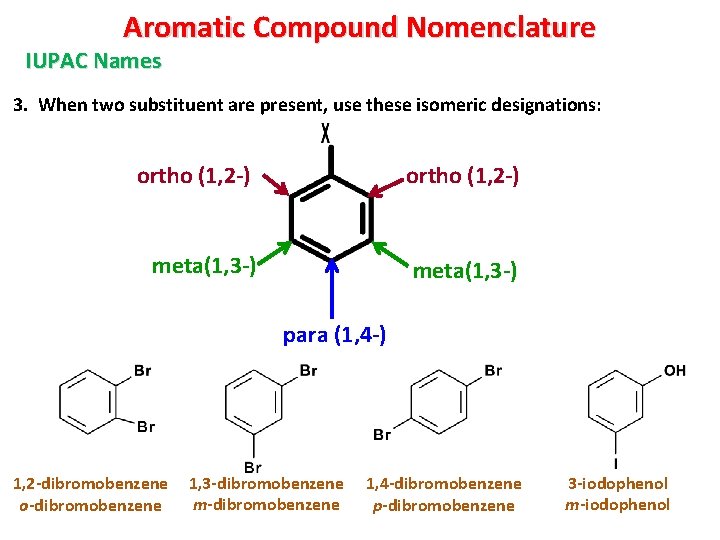
Aromatic Compound Nomenclature IUPAC Names 3. When two substituent are present, use these isomeric designations: ortho (1, 2 -) meta(1, 3 -) para (1, 4 -) 1, 2 -dibromobenzene o-dibromobenzene 1, 3 -dibromobenzene m-dibromobenzene 1, 4 -dibromobenzene p-dibromobenzene 3 -iodophenol m-iodophenol
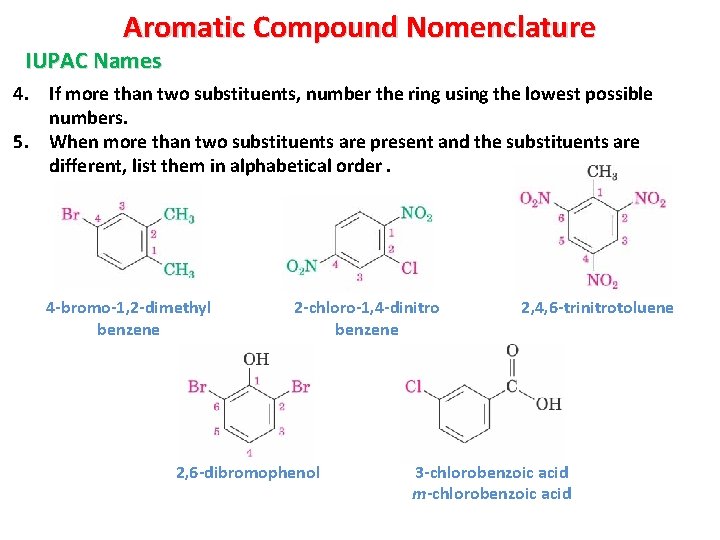
Aromatic Compound Nomenclature IUPAC Names 4. If more than two substituents, number the ring using the lowest possible numbers. 5. When more than two substituents are present and the substituents are different, list them in alphabetical order. 4 -bromo-1, 2 -dimethyl benzene 2 -chloro-1, 4 -dinitro benzene 2, 6 -dibromophenol 2, 4, 6 -trinitrotoluene 3 -chlorobenzoic acid m-chlorobenzoic acid

REACTIONS OF AROMATIC COMPOUNDS
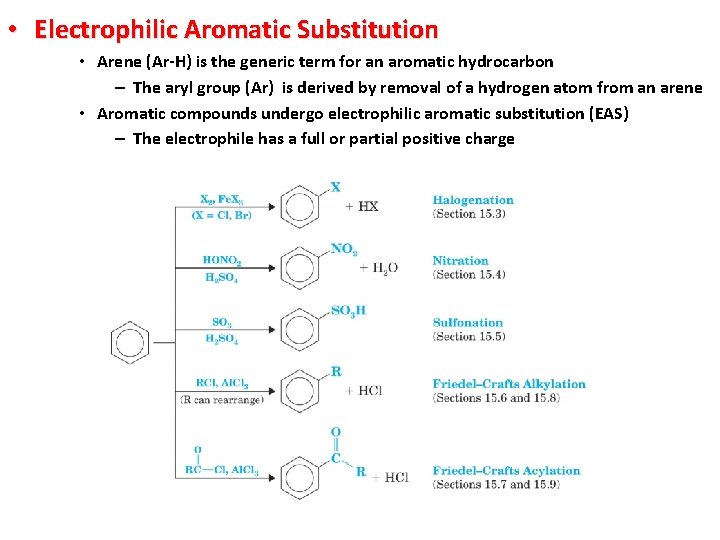
• Electrophilic Aromatic Substitution • Arene (Ar-H) is the generic term for an aromatic hydrocarbon – The aryl group (Ar) is derived by removal of a hydrogen atom from an arene • Aromatic compounds undergo electrophilic aromatic substitution (EAS) – The electrophile has a full or partial positive charge
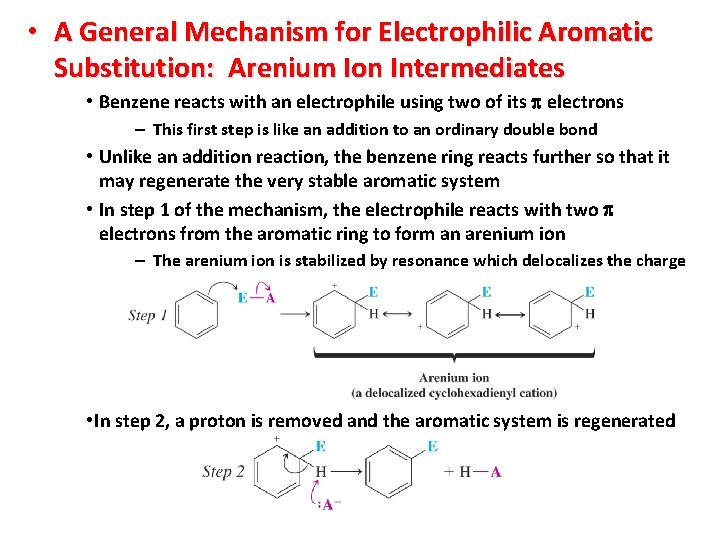
• A General Mechanism for Electrophilic Aromatic Substitution: Arenium Ion Intermediates • Benzene reacts with an electrophile using two of its electrons – This first step is like an addition to an ordinary double bond • Unlike an addition reaction, the benzene ring reacts further so that it may regenerate the very stable aromatic system • In step 1 of the mechanism, the electrophile reacts with two electrons from the aromatic ring to form an arenium ion – The arenium ion is stabilized by resonance which delocalizes the charge • In step 2, a proton is removed and the aromatic system is regenerated
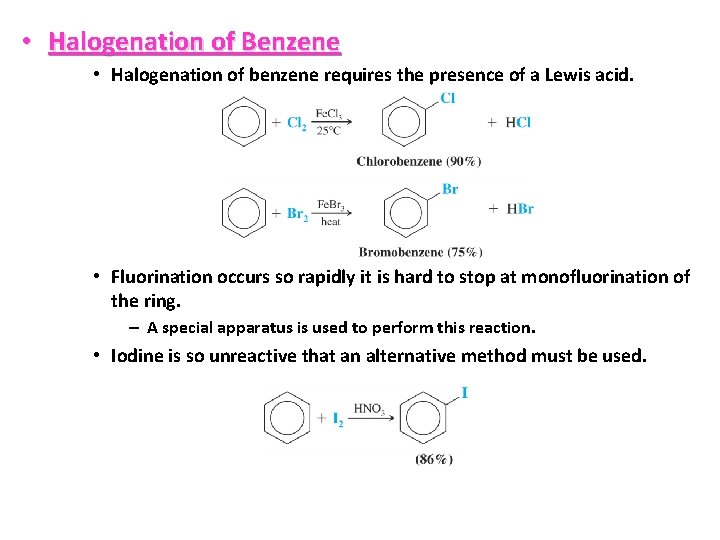
• Halogenation of Benzene • Halogenation of benzene requires the presence of a Lewis acid. • Fluorination occurs so rapidly it is hard to stop at monofluorination of the ring. – A special apparatus is used to perform this reaction. • Iodine is so unreactive that an alternative method must be used.
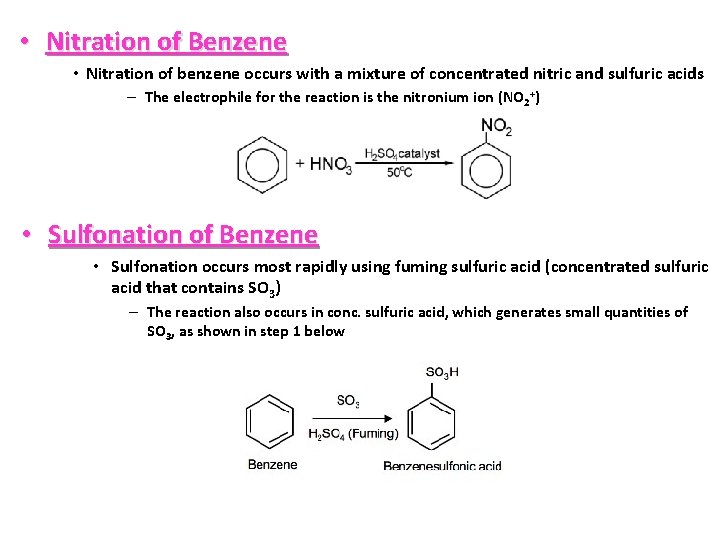
• Nitration of Benzene • Nitration of benzene occurs with a mixture of concentrated nitric and sulfuric acids – The electrophile for the reaction is the nitronium ion (NO 2+) • Sulfonation of Benzene • Sulfonation occurs most rapidly using fuming sulfuric acid (concentrated sulfuric acid that contains SO 3) – The reaction also occurs in conc. sulfuric acid, which generates small quantities of SO 3, as shown in step 1 below
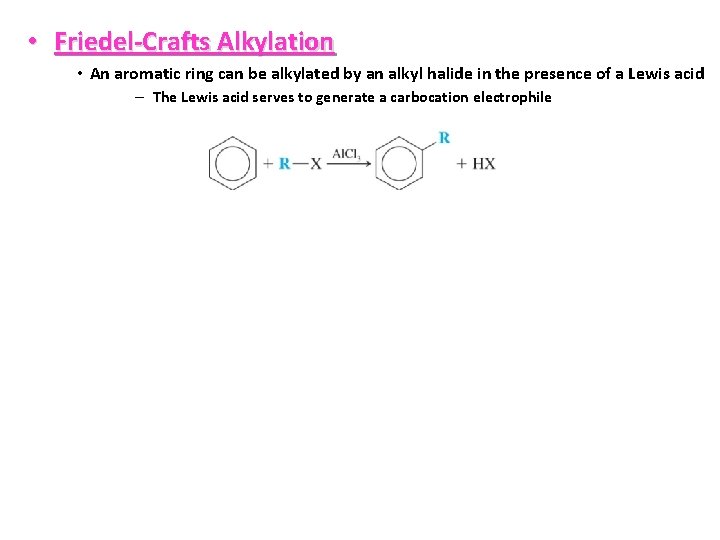
• Friedel-Crafts Alkylation • An aromatic ring can be alkylated by an alkyl halide in the presence of a Lewis acid – The Lewis acid serves to generate a carbocation electrophile
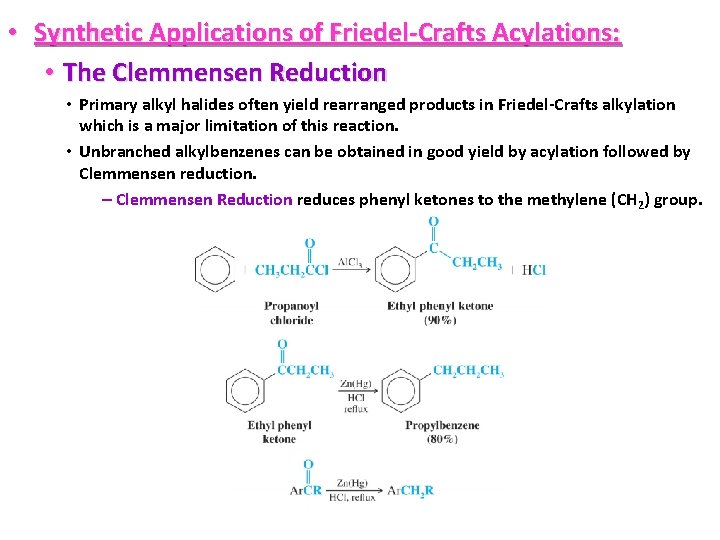
• Synthetic Applications of Friedel-Crafts Acylations: • The Clemmensen Reduction • Primary alkyl halides often yield rearranged products in Friedel-Crafts alkylation which is a major limitation of this reaction. • Unbranched alkylbenzenes can be obtained in good yield by acylation followed by Clemmensen reduction. – Clemmensen Reduction reduces phenyl ketones to the methylene (CH 2) group.
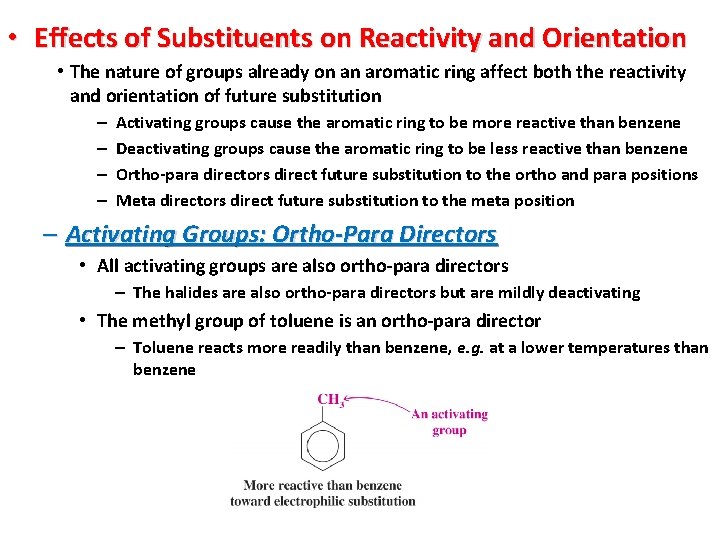
• Effects of Substituents on Reactivity and Orientation • The nature of groups already on an aromatic ring affect both the reactivity and orientation of future substitution – – Activating groups cause the aromatic ring to be more reactive than benzene Deactivating groups cause the aromatic ring to be less reactive than benzene Ortho-para directors direct future substitution to the ortho and para positions Meta directors direct future substitution to the meta position – Activating Groups: Ortho-Para Directors • All activating groups are also ortho-para directors – The halides are also ortho-para directors but are mildly deactivating • The methyl group of toluene is an ortho-para director – Toluene reacts more readily than benzene, e. g. at a lower temperatures than benzene
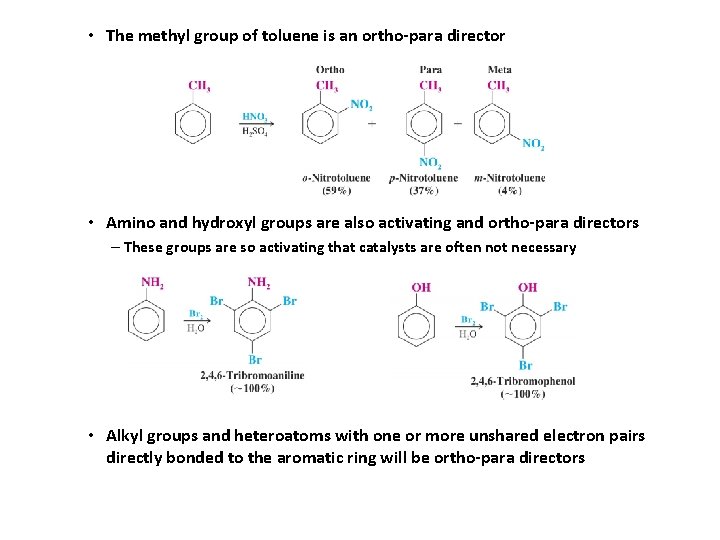
• The methyl group of toluene is an ortho-para director • Amino and hydroxyl groups are also activating and ortho-para directors – These groups are so activating that catalysts are often not necessary • Alkyl groups and heteroatoms with one or more unshared electron pairs directly bonded to the aromatic ring will be ortho-para directors
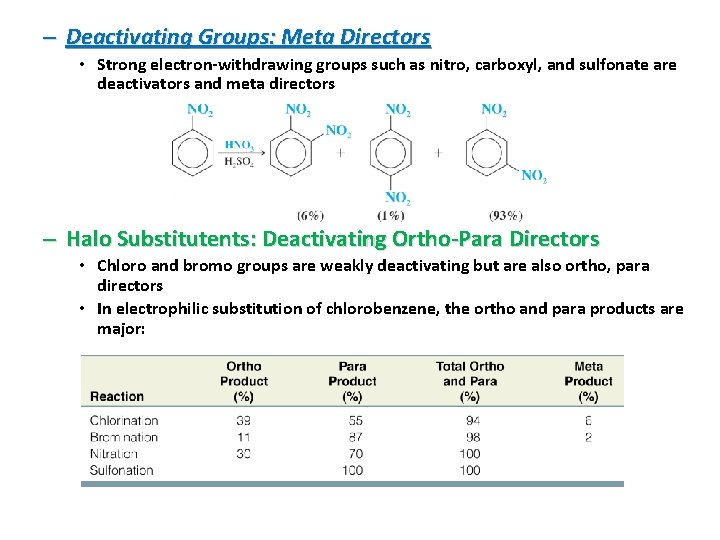
– Deactivating Groups: Meta Directors • Strong electron-withdrawing groups such as nitro, carboxyl, and sulfonate are deactivators and meta directors – Halo Substitutents: Deactivating Ortho-Para Directors • Chloro and bromo groups are weakly deactivating but are also ortho, para directors • In electrophilic substitution of chlorobenzene, the ortho and para products are major:
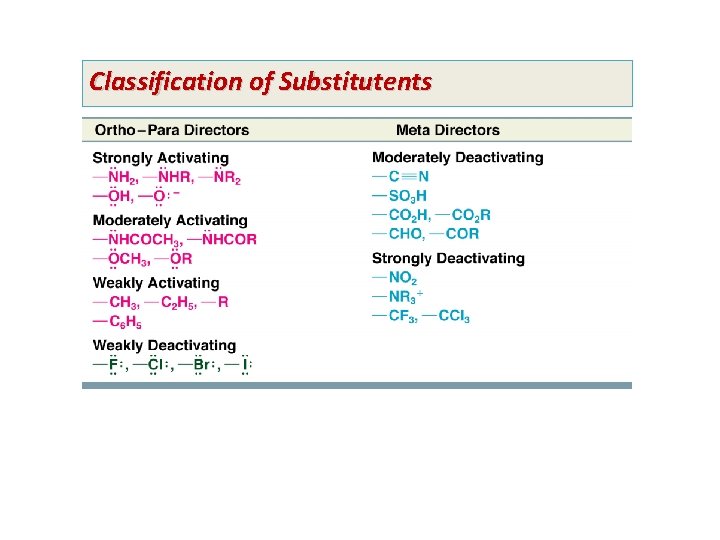
Classification of Substitutents
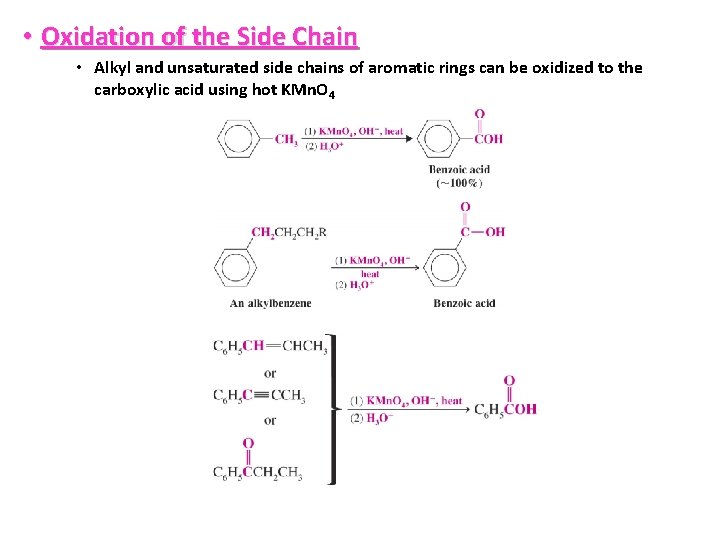
• Oxidation of the Side Chain • Alkyl and unsaturated side chains of aromatic rings can be oxidized to the carboxylic acid using hot KMn. O 4
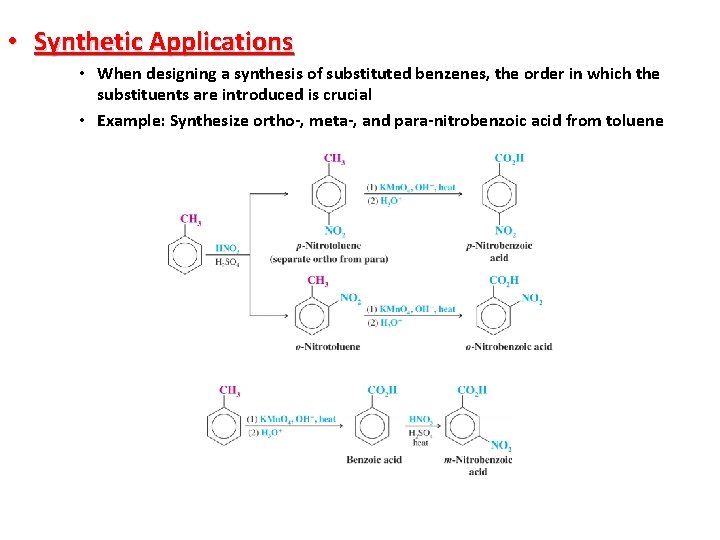
• Synthetic Applications • When designing a synthesis of substituted benzenes, the order in which the substituents are introduced is crucial • Example: Synthesize ortho-, meta-, and para-nitrobenzoic acid from toluene
- Slides: 29