The differences Spinal anesthesia Requires a small mass


















































































































- Slides: 180


The differences: Spinal anesthesia Requires a small mass (volume) of drug, virtually devoid of systemic pharmacologic effect. Epidural anesthesia Necessitates use of a large mass of local anesthetic that produces pharmacologically active systemic blood levels, which may be associated with side effects and complications unknown with spinal anesthesia. Combined spinal & epidural Blurs some of these differences but adds flexibility to critical care.

The use of neuraxial blocks: § Provide postoperative analgesia § Decrease perioperative morbidity § Decrease length of hospital stay § More efficient use of our increasingly health care

Strong contraindication: • Patient refusal • A patient's inability to maintain stillness during the needle puncture • Raised intracranial pressure, which theoretically may predispose to brainstem herniation. • Exposing the neural structures to unacceptable risk of injury

Relative contraindications: • 1 -Must be weighed against the potential benefits include intrinsic and idiopathic coagulopathy, such as that occurring with administration of Coumadin or heparin • 2 -Skin or soft tissue infection at the proposed site of needle insertion

• 3 -Severe hypovolemia • 4 -Lack of anesthesiologist experience • Preexisting neurologic disease (e. g. , lower extremity peripheral neuropathy) (legal)

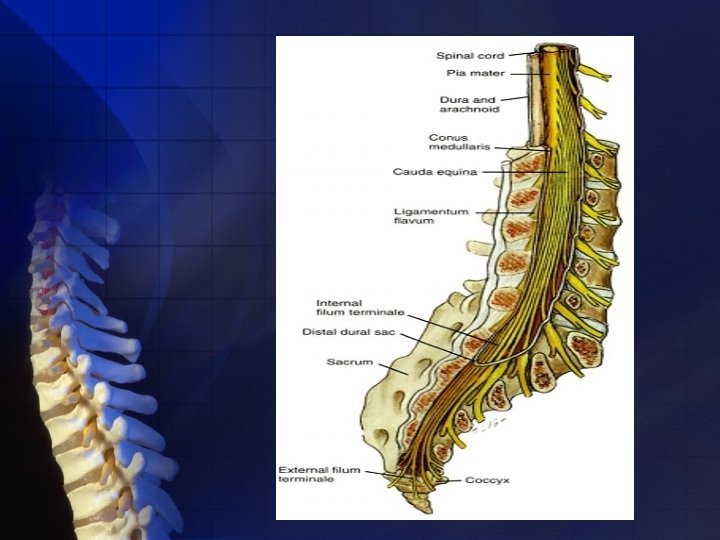
ANATOMY: Subarachnoid L. A. affect their sensory block at the spinal cord, which is continuous cephalad with the brainstem through the foramen magnum and terminates distally in the conus medullaris. This distal termination, because of differential growth rates between the bony vertebral canal and central nervous system (CNS), varies from L 3 in the infant to the lower border of L 1 in the adult.

Dorsal (sensory) roots are generally larger than anterior (motor) roots, the dorsal roots are often blocked more easily. Lumbosacral CSF volume varies from patient to patient, in part because of differences in body habitus and weight. Except for body weight, the volume of CSF does not correlate with other anthropomorphic measurements available clinically.


Surrounding the spinal cord in the bony vertebral column are three membranes (from within to the periphery): • Pia mater • Arachnoid mater • Dura mater

Dur Arachnoid Pia mater a

The pia mater: • Highly vascular membrane • Closely invests the spinal cord and brain The arachnoids mater: • Delicate, nonvascular membrane • Closely attached to the dura • The principal barrier to drugs crossing in and out of the CSF

Dura mater (Theca): • The third and outermost membrane • Fibroelastic membrane • Direct extension of the cranial dura mater • Extends as spinal dura mater from the foramen magnum to S 2, where the filum terminale (an extension of the pia mater beginning at the conus medullaris) blends with the periosteum on the coccyx.

Subarachnoid space contains: • CSF • Spinal nerves • A trabecular network • Blood vessels • Continues to S 2

The subdural space: • A potential space • Between the dura mater and the arachnoid • Contains a small amounts of serous fluid • Not used by anesthesiologists • Due to inject into this space , failed spinal anesthesia happened or rare Total Spinal after epidural anesthesia.

The epidural space: • Surrounding the dura mater • Used by anesthesiologists • Extends from the foramen magnum to the sacral hiatus • Surrounds the dura mater anteriorly, laterally, and more usefully, posteriorly.


The epidural space is bounded: • Anteriorly by the posterior longitudinal ligaments • Laterally by the pedicles the intervertebral foramina • Posteriorly by the ligamentum flavum.

The epidural space in segmented and it’s not uniform. Anatomic dissection and computed tomographic epidurography have also suggested epidural space septa.

Posterior to the epidural space is: • The ligamentum flavum = The yellow ligament v As a single ligament v Composed of two ligamenta flava, the right and the left v Is not uniform from skull to sacrum, nor even within an intervertebral space.


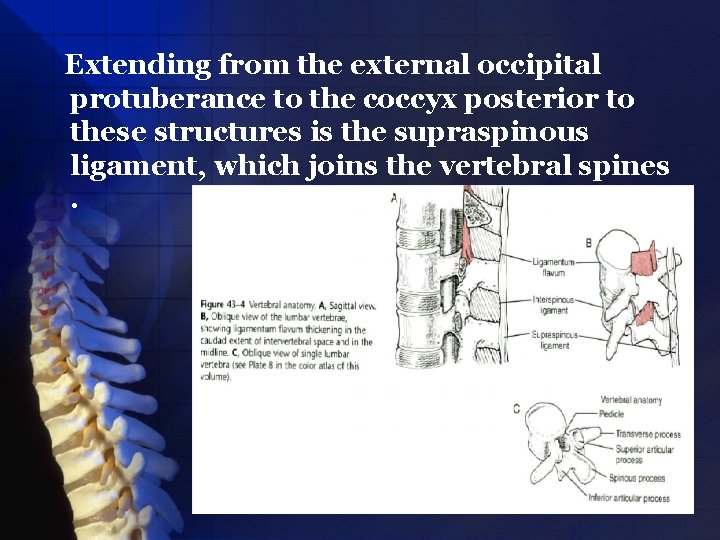
Extending from the external occipital protuberance to the coccyx posterior to these structures is the supraspinous ligament, which joins the vertebral spines.

Contents of the epidural space: • The nerve roots • Fat • Areolar tissue • Lymphatics • Blood vessels which include the well-organized Batson venous plexus.


There is evidence that adipose tissue in the epidural space diminishes with age. Another anatomic change in epidural space anatomy that has long been promoted is that intervertebral foramina decrease in size with increasing age. The decrease in epidural space adipose tissue with age may dominate the age-related changes in epidural dose requirements.


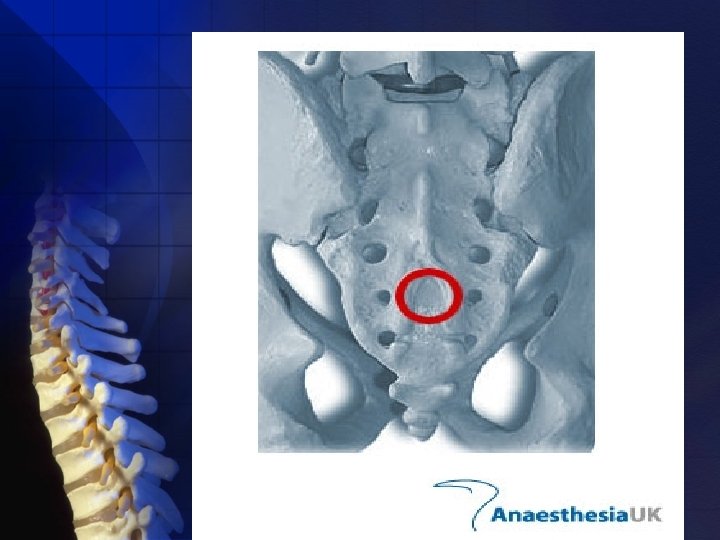
For caudal anesthesia ü ü Epidural anatomy Variations in sacral anatomy Ø The sacrum vertebrae. Ø The sacral hiatus failure of the laminae of S 5 and usually part of S 4 to fuse in the midline. the fusion of the 5 sacral

• Variably shaped. • Sized inverted V shaped bony defect, covered by the posterior sacrococcygeal ligament, a functional counterpart to the ligamentum flava. • May be identified by locating the sacral cornua, the remnants of the S 5 articular processes.




The sacral canal contains: § The terminal portion of the dural sac, which typically ends to a line joining the PSIS, or S 2. § The termination of the dural sac being lower in children, although the ease of palpating the sacral hiatus in children may make pediatric caudal technique easier overall. § A venous plexus which is part of the valveless internal vertebral venous plexus.

It is estimated from magnetic resonance imaging (MRI) studies that the volume of the caudal canal in adults, excluding the foramina and dural sac, is about 10 to 27 m. L. Perhaps this wide variability in volume accounts for some of the variation in block height with caudal anesthesia.

Spinal, epidural, and caudal neuraxial blocks result in: • Sympathetic block • Sensory analgesia • Motor block dose Depending on concentration Of L. A volume

PHYSIOLOGIC EFFECTS (N. A. B): • Cardiovascular Effects • Respiratory Effects • Gastrointestinal Function • Renal Function

Cardiovascular Effects: • Similar to the combined use of IV alpha 1 and beta adrenergic blockers: Decreased HR & BP • The sympathectomy depends on the height of the block as extending for above two to six dermatomes the sensory level with spinal anesthesia and at the same level with epidural anesthesia.

The sympathectomy causes venous and arterial vasodilation. « • If normal cardiac output: Total peripheral resistance 15% to 18% in normovolemic healthy patients.

« In elderly patients with cardiac disease: • SVR 25% after spinal anesthesia • Cardiac output 10%. • Heart rate during high neuraxial block typically decreases blockade of the cardioaccelerator fibers arising from T 1 to T 4.

Heart rate in right atrial filling. Kety and colleagues: Spinal. A. to midthoracic levels with procaine……. . In patients with essential hypertension: - 26% mean arterial pressure (MAP) - 12% cerebral blood flow (CBF).

• The extraction of oxygen was unchanged because myocardial work, as expressed by myocardial use of oxygen, paralleled the decrease in mean arterial pressure and coronary blood flow.

ØDuring T 10 block, there was no significant change in organ blood flow. ØDuring T 1 block 22% decrease in mean arterial pressure(MAP), cerebral and myocardial blood flows(CBF) were insignificantly altered.

After arterial BP decreases to a level for which treatment is necessary( mean arterial pressure more than 30%), ephedrine, a mixed adrenergic agonist, provides more appropriate therapy for the noncardiac circulatory sequelae of neuraxial block than a pure a-adrenergic agonist. Decrease in BP will be minimized by administration of crystalloids intravenously before the block.

« 250 to 2000 ml preblock hydration regimens appear to temporarily increase preload and cardiac output without consistently increasing arterial pressure or preventing hypotension.

Respiratory Effects: Tidal volume Vital capacity 3. 73 L. unchanged during high spinal anesthesia decreases a small amount from 4. 05 to vital capacity in expiratory reserve volume related to paralysis of abdominal muscles necessary forced exhalation, rather than a decrease in phrenic or diaphragmatic function.

The rare respiratory arrest associated with spinal anesthesia is also unrelated to phrenic or inspiratory dysfunction, but rather to hypoperfusion of the respiratory centers in the brainstem.

Neuraxial block should be used cautiously in respiratory cripples because of paralysis of respiratory muscles. Except for the severely compromised patient with respiratory failure, inspiratory muscle function during neuraxial blocks should be adequate to maintain ventilatory function.

Gastrointestinal Function: • Nausea and vomiting in up to 20% of patients related to GI hyperperistalsis due to unopposed parasympathetic (vagal) activity. • Atropine is effective in treating nausea associated with high (T 5) Spinal. • This gastrointestinal hyperperistalsis has the advantage of providing excellent surgical conditions because of a contracted gut.

• The decrease in hepatic blood flow during spinal anesthesia parallels the decrease in MAP. • When epidural analgesia is continued into the postoperative period, there may be a protective effect on the gastric mucosa because intra mucosal PH is higher during postoperative epidural analgesia than with systemic analgesia.

Renal Function: • Despite predictable decreases in renal blood flow accompanying neuraxial block, the decrease is of little physiologic importance. • Neuraxial blocks are a frequent cause of urinary retention, which delays discharge of outpatients and necessitates etebladder cathrization in inpatients.

• Lower concentrations of local anesthetic are necessary for paralysis of bladder function than for motor nerves to lower extremities. • It is prudent to avoid administration of excessive volumes of crystalloid solutions intravenously to patients undergoing spinal anesthesia.

Effects Specific to Epidural Anesthesia The physiologic effects of epidural anesthesia are similar to those of spinal anesthesia, The exception: • L. A. blood levels reach concentrations sufficient enough to produce systemic effects on their own.

• Even intravenously administered lidocaine (resulting in blood levels similar to those after continuous epidural analgesia) can decrease postoperative narcotic requirements. • When blood levels are excessive, adverse CNS and cardiovascular effects occur.

SPINAL ANESTHESIA

Technique To perform spinal anesthesia • Pertinent anatomy must be constantly kept in mind while inserting the spinal needle. • Series of steps ( the four Ps): v Preparation v Position v Projection v Puncture

Preparation equipment drugs The duration of block should be matched to the surgical procedure and to patient variables. • Patient planning to go home after an outpatient procedure, dictating a shorter-acting drug. • A prolonged period of lower extremity analgesia, such as that obtained from adding an opioid to the spinal drugs. When choosing equipment the initial choice involves reusable or disposable equipment.

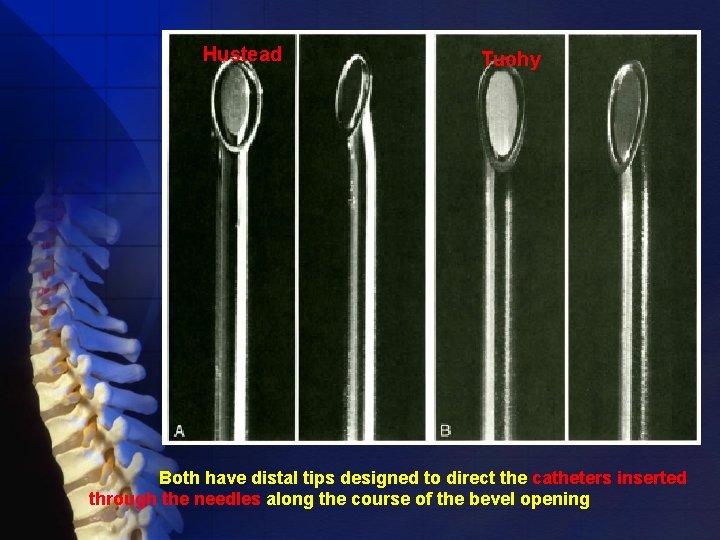
Cut the dura: Quincke- Babcock needle Spinal needles Spread dural fibers: Whitacre and Sprotte needles If a continuous spinal technique is chosen, the use of a Tuohy or other thin-walled needle can facilitate passage of the catheter.





• Use of small needles the incidence PDPH • The use of larger needles improves the tactile sense of needle placement. • Multiple punctures the incidence of headaches. • If use of a smaller needle increases the number of punctures, the difference between small and large needles in producing headaches may be reduced. • Conical-tipped needle the incidence of PDPH

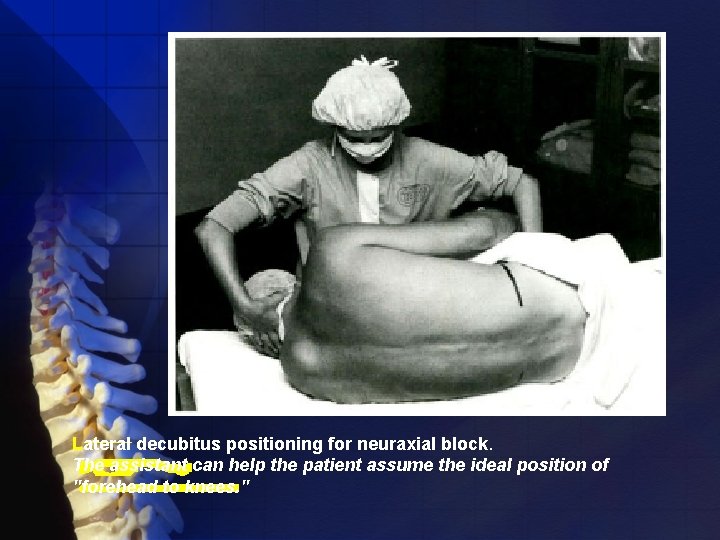
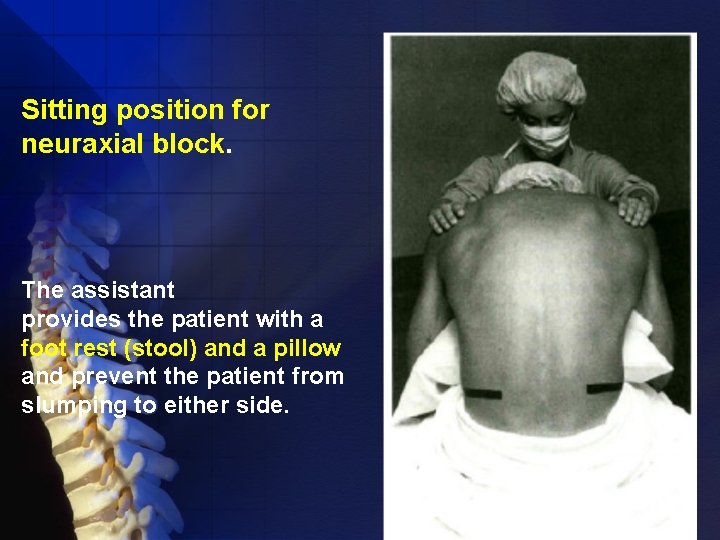
Position • Lateral decubitus (most commonly used) • Sitting • Prone

Lateral decubitus positioning for neuraxial block. The assistant can help the patient assume the ideal position of "forehead to knees. "

Sitting position for neuraxial block. The assistant provides the patient with a foot rest (stool) and a pillow and prevent the patient from slumping to either side.

The sitting position • Low lumbar and sacral levels of sensory adequate for the surgical procedure, such as perineal and urologic operations. • When obesity or scoliosis makes identification of midline anatomy difficult in the lateral position.

When placing patients in this position: • To keep the sensory level low maintained sitting for 5 min (Saddle Anes. ) • If obesity or scoliosis + a higher sensory level is needed, the patient should be put supine immediately after subarachnoid injection, with the table manipulated appropriately.

The prone position • Should be chosen when the patient is to be maintained in that position (often with jackknife modification) during the surgical procedure. • This position is often appropriate for rectal, perineal, or lumbar procedures.

• An advantage of hypobaric technique is that patients can help in positioning themselves, minimizing the opportunity for positioning injuries. • After the patient is in position(PRONE): - Lumbar lordosis minimized - The paramedian approach - Aspirate for CSF

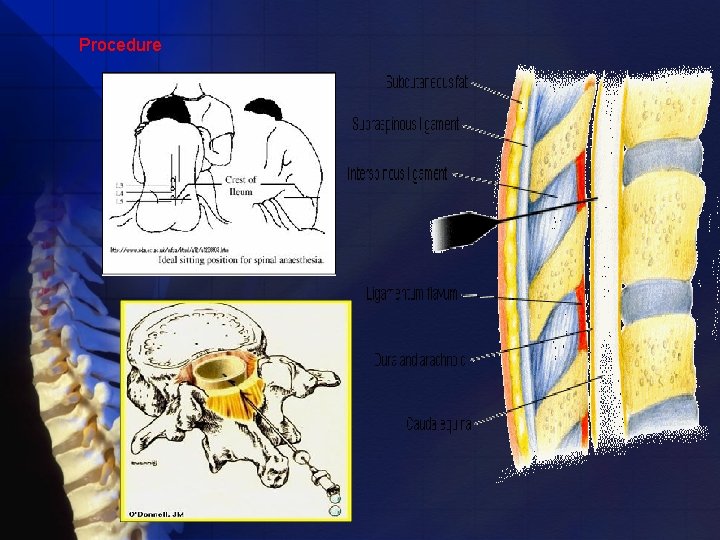
Projection and Puncture * Midline * Paramedian The midline approach • Ability of patients and assistants to minimize lumbar lordosis • Allow access to the subarachnoid space between adjacent spinous processes(L 2 -3, L 3 -4, or L 4 -5 space). • Provides avascular plane.

Skin wheal

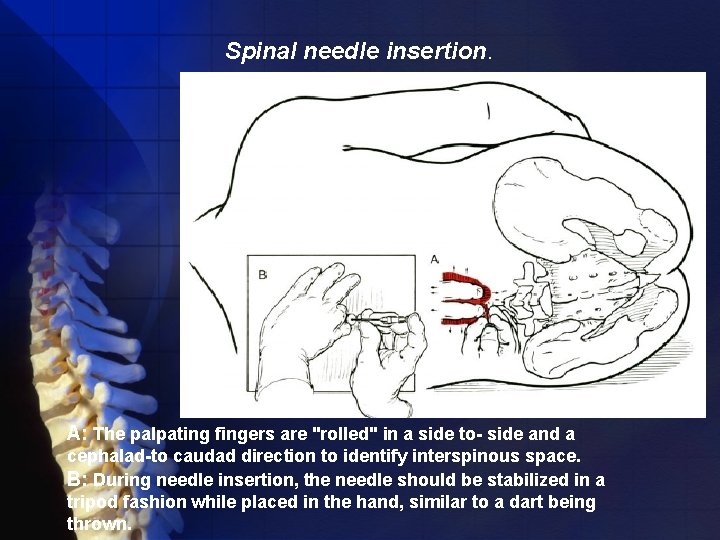
Spinal needle insertion. A: The palpating fingers are "rolled" in a side to- side and a cephalad-to caudad direction to identify interspinous space. B: During needle insertion, the needle should be stabilized in a tripod fashion while placed in the hand, similar to a dart being thrown.

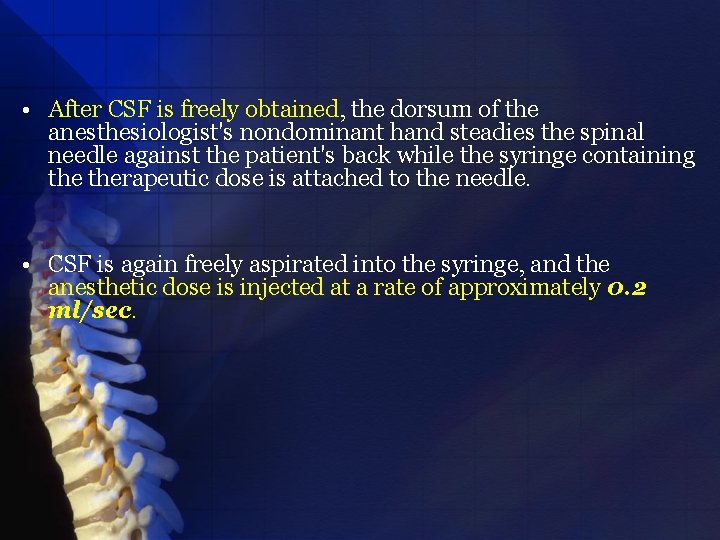
• After CSF is freely obtained, the dorsum of the anesthesiologist's nondominant hand steadies the spinal needle against the patient's back while the syringe containing therapeutic dose is attached to the needle. • CSF is again freely aspirated into the syringe, and the anesthetic dose is injected at a rate of approximately 0. 2 ml/sec.




Procedure

• After completion of the injection, 0. 2 m. L of CSF is aspirated into the syringe and reinjected subarachnoid to reconfirm location and to clear the needle of the remaining local anesthetic. • The patient and operating table should then be placed in the position appropriate for the surgical procedure and drugs chosen.

The paramedian approach • The larger "subarachnoid target" • The most common error: needle entry site is placed too far off the midline (the vertebral lamina barriers to needle insertion). • Identify the caudad edge of the cephalad spinous process, and a skin wheal is raised 1 cm lateral and 1 cm caudad to this point.

• A longer needle (e. g. , 1. 5 to 2 inches) is then used to infiltrate deeper tissues in a cephalomedial plane. • The spinal introducer and needle are then inserted 10 to 15 degrees off the sagittal plane in a cephalomedial plane.

Vertebral anatomy of the midline and paramedian approaches to centroneuraxis blocks

Taylor method: • A variation on the paramedian approach • Lumbosacral approach • At the L 5 -S 1 interspace, the largest interlaminar interspace of the vertebral column. • A 5 -inch spinal needle is inserted in a cephalomedial direction through a skin wheal raised 1 cm medial and 1 cm caudad to the lowermost prominence of the posterosuperior iliac spine (PSIS).

• If bone is encountered on first needle insertion, the needle is walked off the sacrum into subarachnoid space. • After CSF is obtained, the steps are similar to those previously outlined.

Neuraxial anatomy of the Taylor approach to spinal anesthesia.

Continuous spinal anesthetia • A needle with a lateral-faced opening • A midline or paramedian approach (paramedian approach facilitates catheter insertion) • The catheter should be threaded 2 to 3 cm into the subarachnoid space, and then the needle is withdrawn over the catheter.

• Care must be taken to ensure that the catheter is not inserted more deeply into the subarachnoid space when the needle is withdrawn over the catheter. • Catheter-over-the-needle devices are also available for use with continuous spinal anesthesia. • They are(Catheter-over-the-needle) promoted as minimizing the leak of CSF around the catheter.

Hustead Tuohy Both have distal tips designed to direct the catheters inserted through the needles along the course of the bevel opening

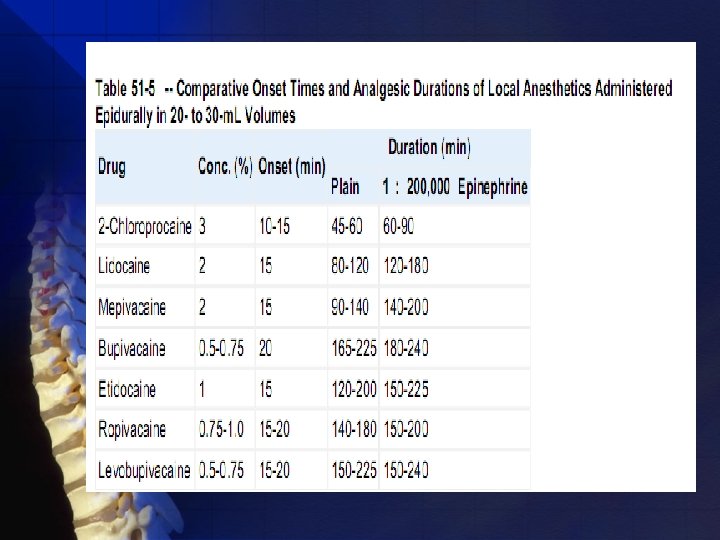
Pharmacology: Useful Drugs Many choice of drugs (in US): procaine (Novocain), lidocaine (Xylocaine), mepivacaine (Carbocaine), tetracaine (Pontocaine), ropivacaine (Neuropin), (S)-(-)-levobupivacaine (Chirocaine), and bupivacaine (Marcaine or Sensorcaine). These drugs provide spinal anesthetics that range from 45 to 400 minutes. • shorter (<90 minutes) • longer (>90 minutes)

Short acting drugs Procaine • One of the oldest spinal anesthetics • Originally replaced cocaine as the drug of choice for spinal anesthesia early in the 20 th century. • It is used for brief spinal anesthetics (<1 hour)

It has some clinical differences with lidocaine: • Higher frequency of nausea (unexplained cause) • A relatively high anesthetic failure rate • Slower time to recovery • The lower frequency of back and leg pain after its use compared with lidocaine. It is often used as a hyperbaric drug in a dose ranging between 50 and 150 to 200 mg in a 10% concentration.

Lidocaine • Shorter procedures can be completed in 1. 5 hours or less • Effect in less than 5 minutes and is most commonly used as the 5% solution in 7. 5% dextrose • It is not clear that reducing the concentration of lidocaine affects the incidence of the back and leg pain that follows its use for spinal anesthesia in what is called transient neurologic symptoms (TNS) and formerly was called transient radicular irritation. • TNS develop most frequently after ambulatory procedures, especially in patients placed in lithotomy or positions for knee arthroscopy.

Use of lidocaine for spinal anesthesia by the following suggestions: § Limit the dose to 60 to 70 mg, • Inject the dose at a rate exceeding 0. 2 ml/sec • Keep the needle aperture directed cephalad • Limit the use of the drug for continuous spinal techniques

Mepivacaine • Used in settings in which lidocaine was used in the past • The drug-mass ratio for mepivacaine and lidocaine is approximately 1. 3: 1, suggesting mepivacaine can be used for spinal anesthesia in a 30 - to 60 -mg dose and typically in the 2%concentration. • Slightly longer-acting lidocaine • Variable reports of lower or equivalent rates of TNS compared with lidocaine.

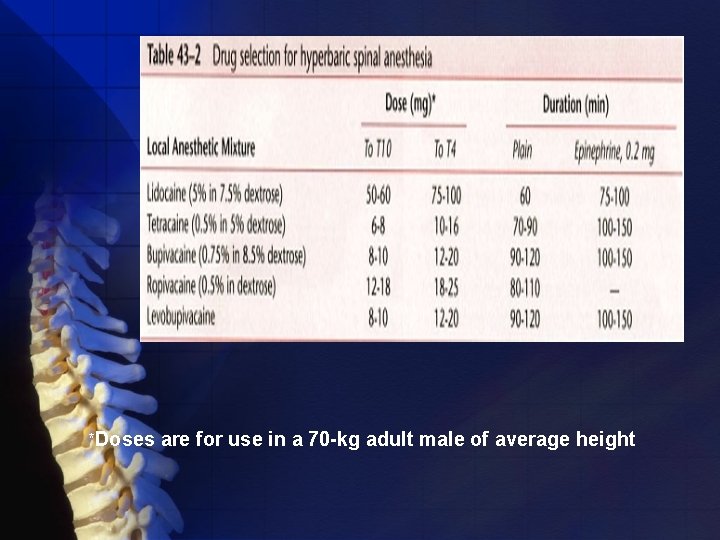
Long acting drugs Tetracaine • Packaged as niphanoid crystals (20 mg) and as a 1% solution (20 mg). • This drug has an onset of 5 to 10 minutes +Epinephrine Up to 2 -3 hours • For procedures +Phenylephrine Up to 5 hours • Epinephrine and phenylephrine prolong spinal anesthesia with tetracaine.

NOTICE: *Duration of blockade is defined as that time between administration of the spinal anesthesia and regression of the blockade to Tl 0 or L 1.

Bupivacaine • 0. 75% and 0. 5% solutions in dextrose • Isobaric form (the 0. 5% and the 0. 75% plain solutions) • The mass of drug (milligram dose) injected is more important in determining the eventual block height than the volume of isobaric drug administered. • Appropriate for procedures lasting up to 2. 5 hours.

Ropivacaine • An amide local anesthetic • Used frequently for epidural anesthesia • Less effect than bupivacaine on the cardiac conduction system. • Compared with bupivacaine, it is estimated to require 1. 8 to 2 times the dose

Levobupivacaine • Isolated (S)-enantiomer of bupivacaine • Available for use as a spinal anesthetic • This drug is bupivacaine when used for spinal anesthesia, and for doses ranging from 4 to 12 mg, • The advantage of levobupivacaine over bupivacaine appears more theoretical than real.

Spinal Anesthetic Additives • Epinephrine (0. 2 mg) • • Phenylephrine (5 mg) (epinephrine (0. 2 mg) or phenylephrine (5 mg) does not decrease spinal cord blood flow in dogs). Clonidine, alpha-2 agonist prolongs motor block associated with tetracaine spinal anesthesia mechanism: vasoconstriction and antinociception from a-stimulation. • Neostigmine acetylcholinesterase inhibitor inhibits the breakdown of acetylcholine that induces analgesia. release of nitric oxide in the spinal cord. side effect of nausea and prolongation of motor block

*Doses are for use in a 70 -kg adult male of average height

Traditionally, epinephrine was thought to prolong only tetracaine spinal anesthesia but not bupivacaine or lidocaine spinal anesthesia Differences: vasodilatory actions of L. A. - Plain lidocaine and bupivacaine vasodilation -Plain tetracaine

In summary : • Epinephrine at 0. 5 mg • Phenylephrine at 5 mg Showed that phenylephrine prolonged tetracaine spinal anesthesia significantly more than epinephrine Phenylephrine prolong lidocaine spinal anesthesia, similar to with epinephrine. Duration of bupivacaine S. A. does not prolonged. by phenylephrine Special care clinician knows what substance all procedures aseptically

Hypobaric and Isobaric Spinal Anesthesia Density: weight in grams of 1 m. L of the solution at a standard temperature. Specific gravity: density of a solution compared in a ratio with the density of water. Baricity: a ratio comparing the density of one solution to another. If the other solution happens to be water, the baricity =the specific gravity

To make a drug hypobaric to CSF • Having a baricity < 1. 0000 or a SG< 1. 0069 (the mean value of CSF's specific gravity) • The rate of injection(0. 2 ml/s versus 0. 5 ml/s) of hypobaric Tetracaine(0. 2%) influences spread of the drug. • Use warmed 0. 5% Bupivacaine more cephalad spread of sensory level, in contrast to a cold or room temperature drug. • . Another drug has a "clinically" hypobaric spinal drug is 2% lidocaine

Factors Affecting Block Height




Complications • Neurologic changes • Headache after dural puncture (PDPH) • Backache (25% of S. A) • Unexpected cardiac arrests

Neurologic Injury • Cases of cauda equina syndrome associated with small-bore continuous S. A. • Lidocaine • Neurologic change can also occur after GA • The risk-benefit equation of anesthesia

Postoperative Headache Post Dural Puncture Headache (PDPH) • More common complication of S. A Also occurs after: • Myelography • Diagnostic lumbar puncture.

Ch. 58: PDPH has the typical feature: • Worsen by standing or straining • Relieve by lying down

Ch. 58: • • Other cause of headache: Nonspecific headache Migraine HTN Pneumocephalus Infections (Sinusitis, meningitis) Cortical vein thrombosis Intracerebral pathology Caffeine withdrawal


Lower incidence of PDPH by: • Needle bevel parallel with the length of the neuraxis • Cone-shaped spinal needle (expose more inflammatory mediator around the opening) • Noncutting needle tip • Early ambulation

Ch. 58: Treatment of PDPH: • Initially conservative • Intake of oral & IV caffeine & analgesics • Intake of fluid(? ) • Drugs (caffeine, vasopressin, theophylline, sumatriptan, ACTH) • Epidural blood patch

Indication of Epidural patch • Symptoms severe enough to limit activity • Evidence of cranial nerve involvement The effectiveness • CSF pressure • Cerebral vasoconstriction

In epidural blood patch: • 15 ml blood(15 -25 ml in Ch. 58) • Volume Success Rate • The symptoms resolve immediately • • Spreads over a mean distance of 9 spinal segment

• One space below the level of insertion & up to 4 spaces above the site (Ch. 58) • Spread cephalad > cauded direction « Its safety & efficacy >90%

Backache: • 25% of all patients undergo Anes. • Should not be attributed to: Needling of the back Use of lido 5% ü ü

Sudden cardiac arrest: • Lack of monitoring • Lack of treatment physiologic explanation • Notice that severe bradycardia after S. A. is not a new phenomenon.

Clinical Pearls The successful integration of neuraxial blocks: • Anesthesia practice • Supplement their blocks with CNS depressants • Supplementation should not be a marker of failed blocks

Clinical Pearls To prevent prolongation of a patient's recovery room stay: Inpatients þ Their block is receding at 4 dermatomes regression or a spinal level < T 10 þ They are hemodynamically stable þ They are comfortable Outpatients þShould be able to ambulate þWithout orthostatic changes þVoid before their discharge

Clinical Pearls • Intraoperatively, during high S. A. patients occasionally complain about dyspnea. • Not result of inspiratory capacity • Related to loss of chest wall sensation • Patients should raise a hand near the mouth and exhale forcefully • Provide reassurance

Clinical Pearls • Neurologic complication with S. A. Neurologic Consultation • EMG alterations associated with denervation due to neurologic injury takes time (14 to 21 days) to develop in the lower extremities. • The physician should obtain EMG studies early and serially after a potential spinal anesthetic-related lesion.

Epidural Anesthesia • Position • Approach: midline & paramedian • Location: cervical, thoracic, lumbar • Technique • loss-of-resistance technique • hanging-drop technique • Monitoring • Single dose - pain management • Continuous epidural - anesthesia & analgesia • Complication • Contraindication • Common LA for epidural anesthesia & analgesia


Epidural Anesthesia

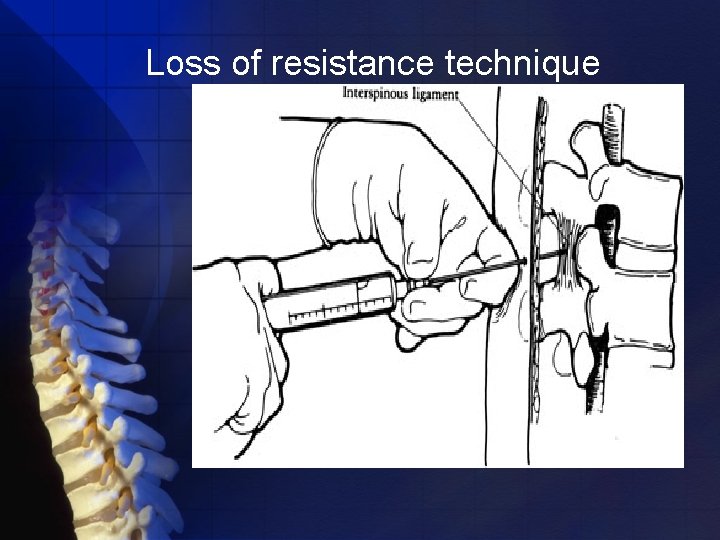
Identification of the epidural space loss-of-resistance technique hanging-drop technique

The needle is grasped with the nondominant hand pulled toward the epidural space while the dominant hand (thumb) applies constant steady pressure on the syringe plunger to compress the air bubble. When the epidural space is entered, the pressure applied to the syringe plunger allows the solution to flow without resistance into the epidural space

The preferred method of carrying out the loss-of-resistance technique involves inserting the needle to the ligamentum flavum and then attaching a 3 - to 5 -m. L glass syringe filled with 2 m. L of saline and a small (0. 25 m. L) air bubble

Loss of resistance technique

An alternative, is the technique of hanging-drop identification of entry into the epidural space. After the needle is placed into the ligamentum flavum, a drop of solution is placed within the hub of the needle. When the needle is advanced into the epidural space, the solution should be “sucked in. ” The theory behind this maneuver has been attributed to subatmospheric pressure in the epidural space. The subatmospheric pressure has been related to expansion of the epidural space as the needle pushes the dura away from the ligamentum flavum

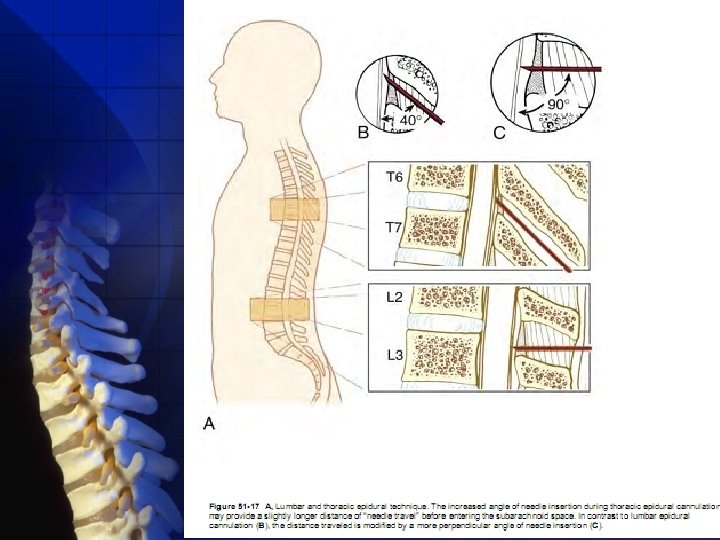
When a lumbar approach is used, the depth from skin to the ligamentum flavum commonly approaches 4 cm , with the depth in most (80%) patients being between 3. 5 and 6 cm. In this region, the ligamentum flavum is 5 to 6 mm thick in the midline ,

Thoracicapproach injury to the spinal cord is possible if the needle is advanced too far. . the increased angle of needle insertion may provide an element of safety in that the more acute angle necessary to gain epidural cannulation provides some margin of safety

thoracic epidural anesthetics do not appear to be associated with an increased incidence of neurologic injury because those choosing to use the technique are most often anesthesiologists with considerable experience in lumbar epidural anesthesia

when the anesthesiologist chooses to cannulate the epidural space with a catheter success may be increased by advancing the needle 1 to 2 mm after the space is identified


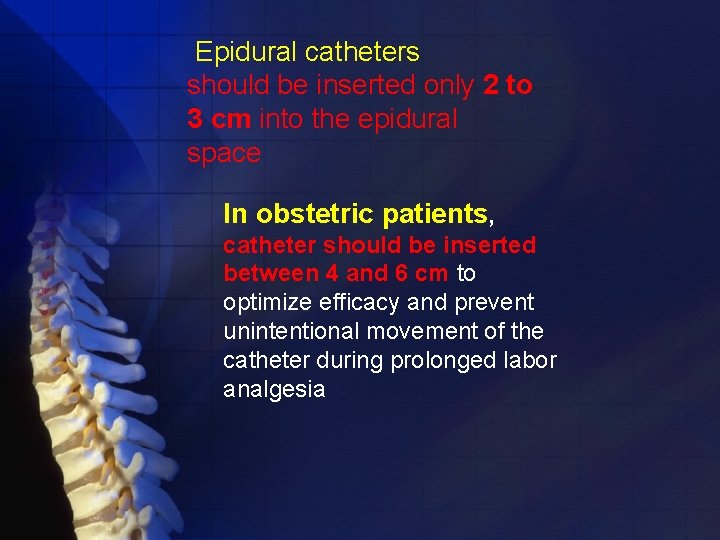
Epidural catheters should be inserted only 2 to 3 cm into the epidural space In obstetric patients, catheter should be inserted between 4 and 6 cm to optimize efficacy and prevent unintentional movement of the catheter during prolonged labor analgesia

Threading more catheter may increase the likelihood of catheter malposition. If malposition does occur, data suggest that one of the more common sites of malposition is entry of the catheter into the anterior epidural space

Despite an adequately positioned catheter during first use of a local anesthetic, each subsequent injection should be preceded by aspiration and an epidural test dose because catheter migration into vessels and the subarachnoid or subdural space does occur




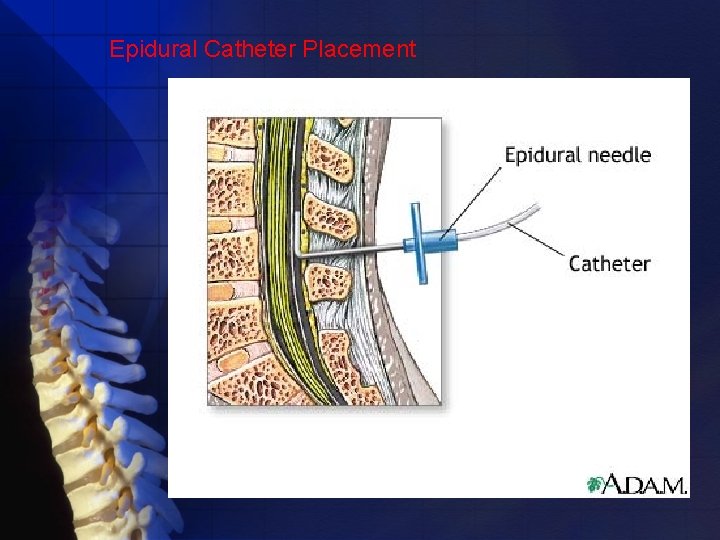
Epidural Catheter placement

Epidural Catheter Placement

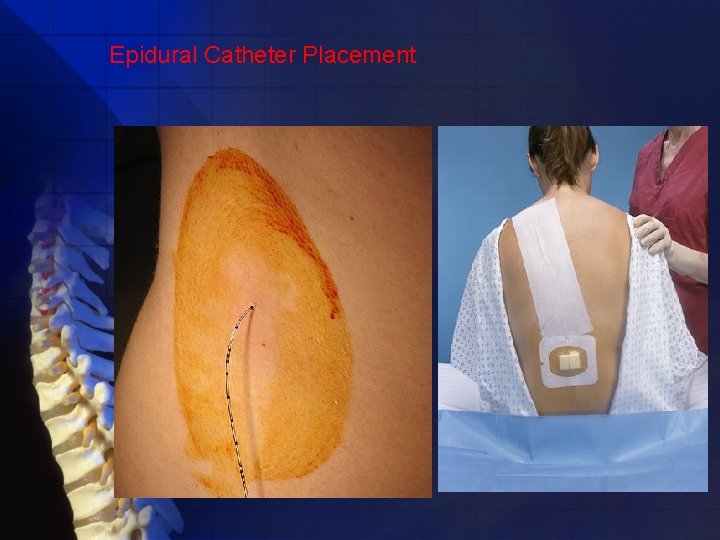
Epidural Catheter Placement

Epidural Catheter Placement

Pharmacology Drugs available for epidural use can be categorized as short intermediate-, and long-acting local anesthetics,


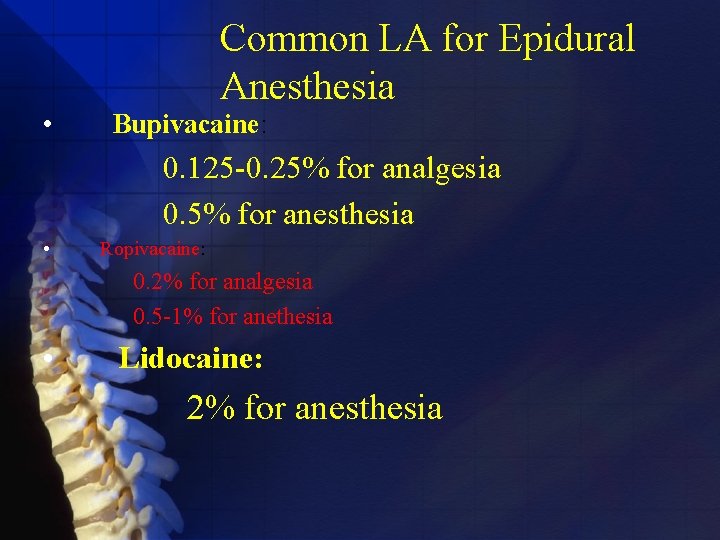
• Common LA for Epidural Anesthesia Bupivacaine: 0. 125 -0. 25% for analgesia 0. 5% for anesthesia • Ropivacaine: 0. 2% for analgesia 0. 5 -1% for anethesia • Lidocaine: 2% for anesthesia

Lidocaine is the prototypical amide local anesthetic and is used epidurally in 1. 5% to 2% concentrations. Mepivacaine is similar to lidocaine in the concentrations necessary for epidural anesthesia and lasts 15 to 30 minutes longer.

Additives to make epidural anesthesia last longer, to improve the quality of blockade, or to accelerate the onset of blockade

Additives 1) Epinephrine 2) Phenylephrine 3) Bicarbonate

Epinephrine increases the duration of useful anesthesia with all the agents, although the proportional effect is greatest with lidocaine, mepivacaine, and 2 -chloroprocaine it shows a lesser effect with bupivacaine, levobupivacaine, and etidocaine and has a limited effect with ropivacaine

Phenylephrine has been used in epidural anesthesia less widely than in spinal anesthesia, perhaps because it does not reduce peak blood levels of local anesthetic as effectively as epinephrine does during epidural use.

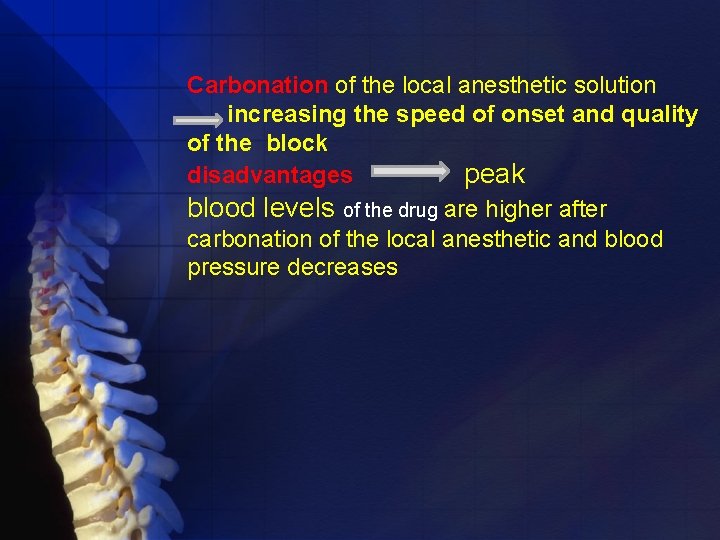
Carbonation of the local anesthetic solution increasing the speed of onset and quality of the block disadvantages peak blood levels of the drug are higher after carbonation of the local anesthetic and blood pressure decreases

The addition of bicarbonate increasing the p. H of the local anesthetic solution and increasing the concentration of nonionized free base, which theoretically increases the rate of diffusion of the drug and the speed of onset of the block.

. Clinically, the addition of 1 m. Eq of sodium bicarbonate to each 10 m. L of 1. 5% lidocaine solution produces a significantly faster onset of anesthesia and more rapid spread of sensory block may be produced

Complications • Similar to spinal anesthesia • Wet tap – postpuncture headache • Total spinal anesthesia – apnea, hypotension, bradycardia

Complications Intravascular Injection Epidural anesthesia has the potential to produce local anesthetic–induced systemic toxicity primarily through the unintentional administration of drug into an epidural vein

The toxic effects of local anesthetics primarily involve the CNS and cardiovascular system,

The use of test doses should minimize unintentional intravascular injections

CNS affected at lower blood levels The CNS stimulation is a result of selective inhibition of inhibitory neurons in the cerebral cortex, which allows facilitatory neurons to producing CNS excitation After blood levels are high enough, inhibitory and facilitatory pathways are inhibited, thereby leading to CNS depression.

the potential for local anesthetic–induced toxicity (CNS and cardiovascular system) was thought to parallel anesthetic potency

CNS toxicity or frank convulsions Early in local anesthetic–induced seizures, hypoxemia, hypercapnia, and acidosis develop rapidly, symptomatic treatment of the toxicity must involve treatment of these factors.

Oxygen should be given by bag and mask, and tracheal intubation is not mandated unless ventilation is Ineffective administration of succinylcholine or an anticonvulsant

Succinylcholine is often recommended because the local anesthetic–induced seizures are usually short-lived and the muscle relaxation obtained facilitates ventilation and decreases the magnitude ofthe metabolic acidosis

Succinylcholine does not decrease cerebral metabolism. CNS oxygen requirements remain increased.

Diazepam is effective in controlling local anesthetic–induced seizures,

The most effective method of treating toxic reactions is prevention Administration of excessive doses of local anesthetics should be prevented, and test doses of local anesthetics should be used before the injection of therapeutic doses.

ABSOLUTE CONTRA INDICATIONS • Patient refusal • Inability of patient to maintain position • Raised ICP Relative contraindication: Coagolopathy –skin or soft tissue infection –sever hypovolemia – lack of anaesthesiologist experience

The risk of neuraxial bleeding is increased when LMWH and neuraxial techniques are combined if the following recommendations are not followed

. Neuraxial block should be delayed for at least 10 to 12 hours after the last dose of LMWH in patients receiving the drug preoperatively. 1

2. Postoperative treatment with LMWH should be delayed at least 12 hours after completion of the surgical procedure.

3. Removal of the epidural and spinal catheters used for postoperative analgesia should take place 10 to 12 hours after the last dose, with subsequent dosing delayed for at least 2 hours.

Caudal Technique Caudal anesthesia requires identification of the sacral hiatus. The sacrococcygeal ligament (i. e. , extension of ligamentum flavum) overlying the sacral hiatus lies between the sacral cornua.

To facilitate locating the cornua, the posterior superior iliac spines should be located and, by using the line between them as one side of an equilateral triangle, the location of the sacral hiatus should be approximated

the caudal needle is inserted at an angle of approximately 45 degrees to the sacrum. While advancing the needle, a decrease in resistance to needle insertion should be appreciated as the needle enters the caudal canal. The needle is advanced until bone (i. e. , dorsal aspect of the ventral plate of the sacrum) is contacted and then slightly withdrawn, and the needle is redirected so that the angle of insertion relative to the skin surface is decreased.

One method of increasing the likelihood of correct caudal needle placement is to inject 5 m. L of saline rapidly through the caudal needle while palpating the skin overlying the sacrum. If no midline bulge is detected, the needle is probably positioned correctly. In contrast, if a midline bulge is detected during saline injection, the needle is positioned incorrectly. .
