The Diabetic Retinopathy Clinical Research Network Dedicated to

The Diabetic Retinopathy Clinical Research Network Dedicated to multicenter clinical research of diabetic retinopathy, macular edema and associated conditions Carl W. Baker, MD for the Diabetic Retinopathy Clinical Research Network Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services EY 14231, EY 018817
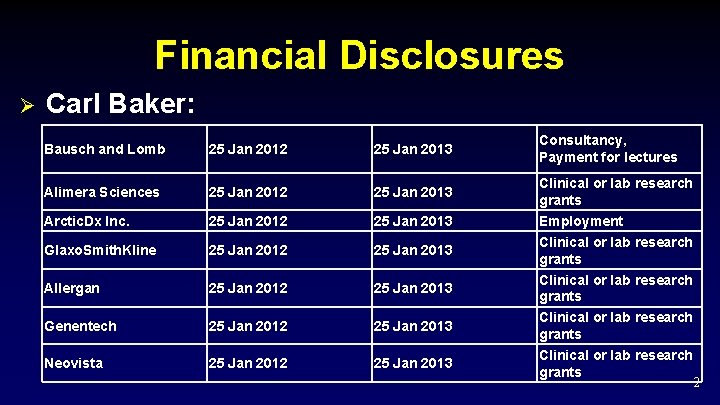
Financial Disclosures Ø Carl Baker: Bausch and Lomb 25 Jan 2012 25 Jan 2013 Alimera Sciences 25 Jan 2012 25 Jan 2013 Arctic. Dx Inc. 25 Jan 2012 25 Jan 2013 Glaxo. Smith. Kline 25 Jan 2012 25 Jan 2013 Allergan 25 Jan 2012 25 Jan 2013 Genentech 25 Jan 2012 25 Jan 2013 Neovista 25 Jan 2012 25 Jan 2013 Consultancy, Payment for lectures Clinical or lab research grants Employment Clinical or lab research grants 2

Course Outline 1. DRCR. net Overview. Carl W. Baker, MD 2. DRCR. net recent trial results § Intravitreal Anti-VEGF vs. Saline for Diabetic Vitreous Hemorrhage. Abdhish R. Bhavsar, MD 3. Rationale for ongoing trials § Comparison of aflibercept, ranibizumab, and bevacizumab for DME. Jack Wells, MD § Ranibizumab vs. PRP for Proliferative diabetic retinopathy. Jennifer K. Sun, MD 3

Course Outline 4. Common Topics for Clinical case review/discussion § § Managing DME and PDR when both Incomplete response of DME to anti present in the same eye. -VEGF treatment. Susan B Bressler, MD § § Standard vs wide-field fundus Asymptomatic 20/20 VA with central photographs and fluorescine angiography -involved DME. in the management of DME and PDR. Neil M Bressler, MD § John A Wells, III MD Role of laser in DME. Lee Jampol, MD Jennifer K Sun, MD § DME treatment in Pseudophakic Eyes. Scott M Friedman, MD 4

DRCR. net Overview Ø Ø Objective: • The development of a collaborative network to facilitate multicenter clinical research on diabetic retinopathy, DME and associated conditions. Funding: • National Eye Institute (NEI) and The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-sponsored cooperative agreement initiated September 2002. o Current award 2009 -2013 5

DRCR. net Overview Ø Network Chair: Neil M. Bressler, M. D. Ø National Eye Institute Project Officer: Eleanor B. Schron, Wilmer Ophthalmological Institute Ph. D. , R. N. at Johns Hopkins, Baltimore, MD Ø Network Chair elect: Lee M. Jampol, M. D. Northwestern University Medical School, Department of Ophthalmology, Chicago, IL Ø Director of the Coordinating Center: Adam R. Glassman, M. S. (Jaeb Center for Health Research) Ø Vice-Chairs (2013): Carl W. Baker, M. D. , Paducah Retinal Center, Scott M. Friedman, M. D. , Florida Retina Consultants Jennifer K. Sun, M. D. , M. P. H. , Joslin Diabetes Center 6

Priority Initiatives Ø Involvement of community-based practices, as well as “academic” or university-based centers. Ø Collaborate with industry to facilitate investigations and pursue opportunities otherwise not possible and to do so in a manner consistent with the Network’s dedication to academic integrity and optimal clinical trial performance. 7

Organization: Clinical Sites of the Network Ø Overall Network Participation (as of 10/07/13) • • Ø 285 sites submitted application for Network 1017 total Investigators; 3261 additional personnel Network is open and continually solicits participation of new sites and investigators 8
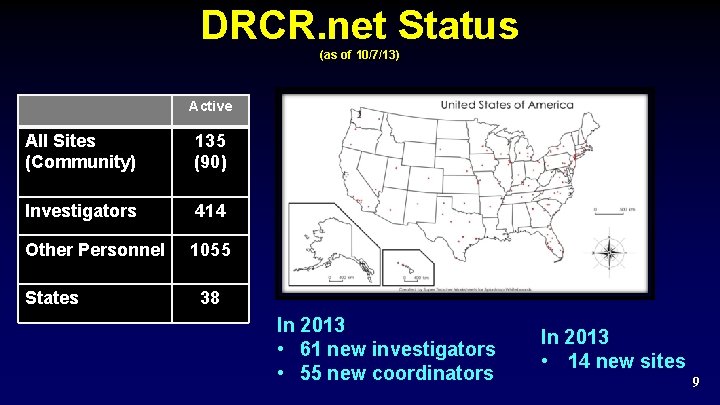
DRCR. net Status (as of 10/7/13) Active All Sites (Community) 135 (90) Investigators 414 Other Personnel 1055 States 38 In 2013 • 61 new investigators • 55 new coordinators In 2013 • 14 new sites 9
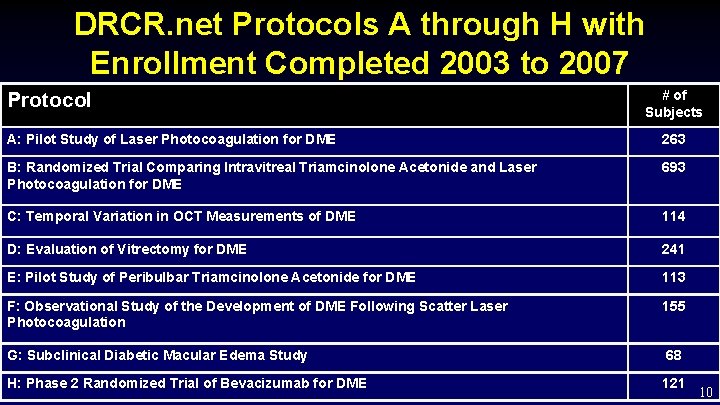
DRCR. net Protocols A through H with Enrollment Completed 2003 to 2007 Protocol # of Subjects A: Pilot Study of Laser Photocoagulation for DME 263 B: Randomized Trial Comparing Intravitreal Triamcinolone Acetonide and Laser Photocoagulation for DME 693 C: Temporal Variation in OCT Measurements of DME 114 D: Evaluation of Vitrectomy for DME 241 E: Pilot Study of Peribulbar Triamcinolone Acetonide for DME 113 F: Observational Study of the Development of DME Following Scatter Laser Photocoagulation 155 G: Subclinical Diabetic Macular Edema Study 68 H: Phase 2 Randomized Trial of Bevacizumab for DME 10 121 10
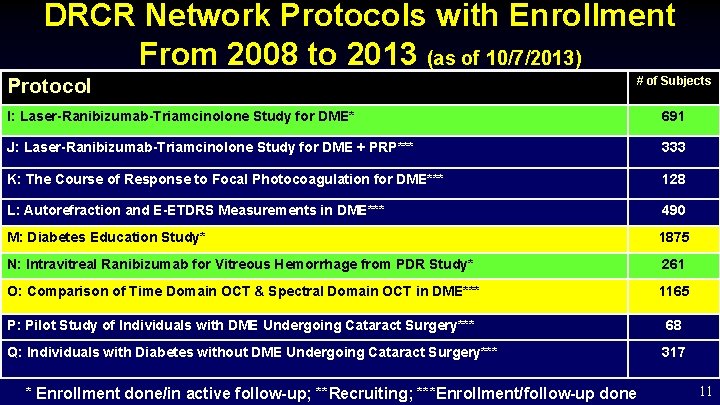
DRCR Network Protocols with Enrollment From 2008 to 2013 (as of 10/7/2013) Protocol # of Subjects I: Laser-Ranibizumab-Triamcinolone Study for DME* 691 J: Laser-Ranibizumab-Triamcinolone Study for DME + PRP*** 333 K: The Course of Response to Focal Photocoagulation for DME*** 128 L: Autorefraction and E-ETDRS Measurements in DME*** 490 M: Diabetes Education Study* 1875 N: Intravitreal Ranibizumab for Vitreous Hemorrhage from PDR Study* 261 O: Comparison of Time Domain OCT & Spectral Domain OCT in DME*** 1165 P: Pilot Study of Individuals with DME Undergoing Cataract Surgery*** 68 Q: Individuals with Diabetes without DME Undergoing Cataract Surgery*** 317 * Enrollment done/in active follow-up; **Recruiting; ***Enrollment/follow-up done 11

DRCR Network Protocols with Enrollment From 2008 to 2012 (as of 9/30/12) Protocol # of Subjects R: NSAIDs in Eyes with Non Central Involved DME* 125 S: Prompt PRP vs. Ranibizumab + Deferred PRP for DPR* 305 T: Anti-VEGF comparison* 660 Genetics Ancillary Study: Genes in Diabetic Retinopathy** 807 V: Anti-VEGF vs, Laser for CI DME in Eyes with Very Good VA** DRCR. Net Participant Total Since 2003 * Enrollment done/in active follow-up; **Recruiting; ***Enrollment/follow-up done 0 7, 630 12
What Has Been Learned? Diabetic Macular Edema Treatment Ø Ø Ø Protocol B: Over 2 years, focal/grid photocoagulation is more effective and has fewer side effects than 1 mg or 4 mg doses of preservative-free intravitreal triamcinolone. Protocol E: In cases of DME with good visual acuity, peribulbar triamcinolone, with or without focal photocoagulation, is unlikely to be of substantial benefit. Protocol H: The results demonstrated that intravitreal bevacizumab can reduce DME in some eyes, but the study was not designed to determine whether the treatment was 13 beneficial.
What Has Been Learned? Diabetic Macular Edema Treatment Ø Ø Protocol I: Intravitreal ranibizumab with prompt or deferred (≥ 24 weeks) focal/grid laser is more effective through 2 years in increasing visual acuity compared with focal/grid laser treatment alone for the treatment of DME involving the central macula. Ranibizumab should be considered for patients with DME and decreased visual acuity. Protocol K: Sixteen weeks after focal/grid laser for diabetic macular edema in eyes with a definite reduction, but not resolution, of central edema, 23% to 63% likely will continue to improve without additional treatment. 14

What Has Been Learned? Diabetic Retinopathy Treatment Ø Ø Protocol F: Clinically meaningful differences are unlikely in OCT thickness or visual acuity following application of PRP in 1 sitting compared with 4 sittings. These results suggest PRP costs to some patients in terms of travel and lost productivity as well as to eye care providers could be reduced. Protocol J: The addition of 1 or 2 intravitreal triamcinolone injections in eyes receiving focal/grid laser for DME and PRP is associated with better visual acuity and decreased macular edema by 14 weeks. Whether continued long-term intravitreal treatment is beneficial cannot be determined from this study. 15
What Has Been Learned? OCT and Retinal Thickening Ø Ø Ø Protocol C: Although on average there are slight decreases in retinal thickening during the day, most eyes with DME have little meaningful change in OCT CSF thickening or VA between 8 AM and 4 PM. Protocol C: Reproducibility of retinal thickness in DME was better for CSF thickness than for center point measurements. A change in CSF thickness exceeding 11% is likely to be real. Protocol G: While subclinical DME may be uncommon, this study suggests that between ~25% and 50% of eyes with subclinical DME will progress to more definite thickening or be judged to need treatment for DME within 2 years after its identification. 16

What Has Been Learned? Optical Coherence Tomography Ø Ø Protocol G: CSF thickness on Stratus OCT™ in people with diabetes and minimal or no retinopathy are similar to a normative database of people without diabetes. CSF thickness is greater in men than in women. Studies involving comparisons of retinal thickness to expected norms should consider different mean values for women and men. Protocol O: Mean CSF thickness is ~70 µm thicker when measured with Heidelberg Spectralis OCT as compared with Stratus OCT among individuals with diabetes in the absence of retinopathy or with minimal non-proliferative retinopathy and a normal macular architecture. CSF thickness values ≥ 320 µm for men and 305 µm for women are proposed 17 as gender-specific thickness levels.
Active Studies Image: National Eye Institute, National Institutes of Health 18
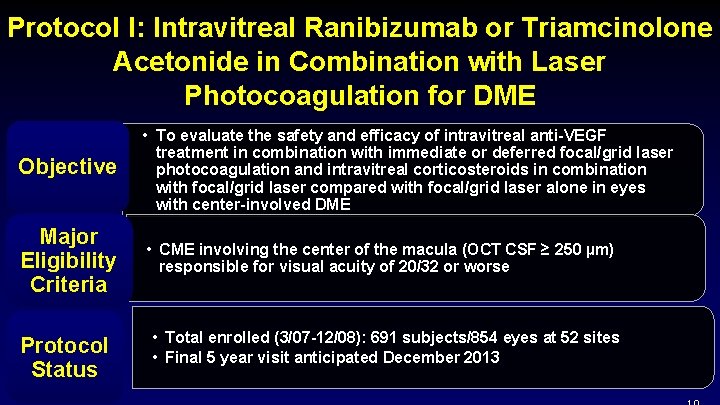
Protocol I: Intravitreal Ranibizumab or Triamcinolone Acetonide in Combination with Laser Photocoagulation for DME Objective • To evaluate the safety and efficacy of intravitreal anti-VEGF treatment in combination with immediate or deferred focal/grid laser photocoagulation and intravitreal corticosteroids in combination with focal/grid laser compared with focal/grid laser alone in eyes with center-involved DME Major Eligibility Criteria • CME involving the center of the macula (OCT CSF ≥ 250 µm) responsible for visual acuity of 20/32 or worse Protocol Status • Total enrolled (3/07 -12/08): 691 subjects/854 eyes at 52 sites • Final 5 year visit anticipated December 2013

Ø Protocol M: Effect of Diabetes Education during Ophthalmology Visits on Diabetes Control Objective • To assess whether glycemic control (assessed with Hb. A 1 c measurement) in individuals with type 1 or type 2 diabetes can be improved with a point-of-care measurement of Hb. A 1 c in the ophthalmologist’s office combined with a personalized risk assessment for diabetic retinopathy and other complications of diabetes 20
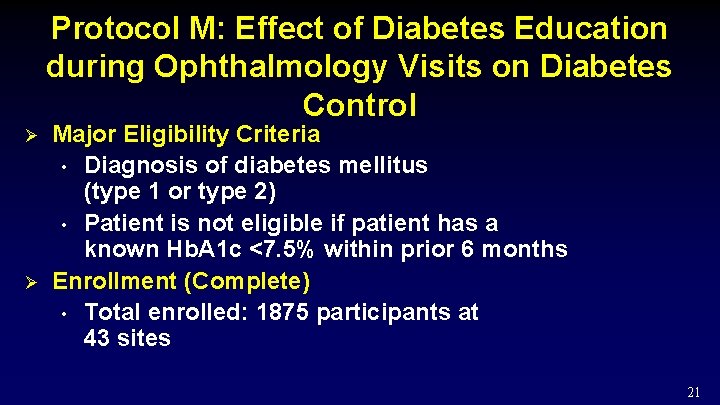
Protocol M: Effect of Diabetes Education during Ophthalmology Visits on Diabetes Control Ø Ø Major Eligibility Criteria • Diagnosis of diabetes mellitus (type 1 or type 2) • Patient is not eligible if patient has a known Hb. A 1 c <7. 5% within prior 6 months Enrollment (Complete) • Total enrolled: 1875 participants at 43 sites 21

Protocol N: An Evaluation of Intravitreal Ranibizumab for Vitreous Hemorrhage Due to Proliferative Diabetic Retinopathy Ø Objective • To determine if intravitreal injections of ranibizumab decrease the proportion of eyes in which vitrectomy is performed compared with saline injections in eyes presenting with vitreous hemorrhage from proliferative diabetic retinopathy 22
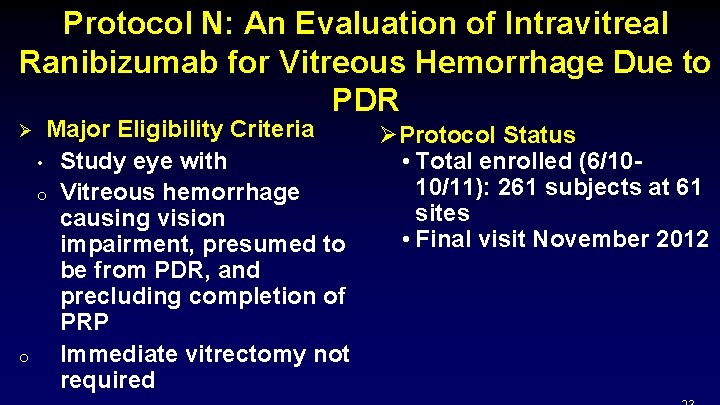
Protocol N: An Evaluation of Intravitreal Ranibizumab for Vitreous Hemorrhage Due to PDR Major Eligibility Criteria ØProtocol Status • Study eye with • Total enrolled (6/1010/11): 261 subjects at 61 o Vitreous hemorrhage sites causing vision • Final visit November 2012 impairment, presumed to be from PDR, and precluding completion of PRP o Immediate vitrectomy not required Ø
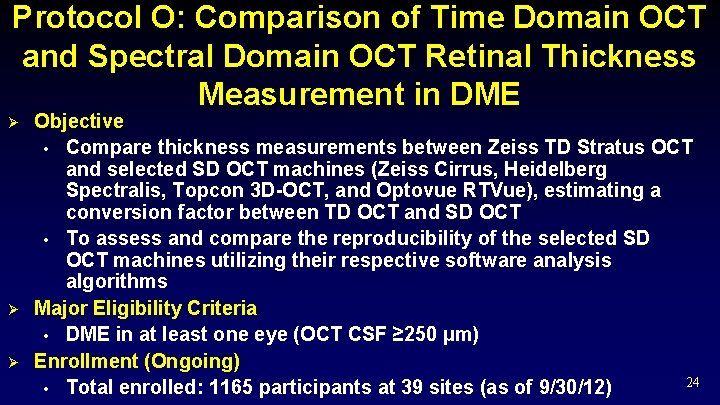
Protocol O: Comparison of Time Domain OCT and Spectral Domain OCT Retinal Thickness Measurement in DME Ø Ø Ø Objective • Compare thickness measurements between Zeiss TD Stratus OCT and selected SD OCT machines (Zeiss Cirrus, Heidelberg Spectralis, Topcon 3 D-OCT, and Optovue RTVue), estimating a conversion factor between TD OCT and SD OCT • To assess and compare the reproducibility of the selected SD OCT machines utilizing their respective software analysis algorithms Major Eligibility Criteria • DME in at least one eye (OCT CSF ≥ 250 µm) Enrollment (Ongoing) 24 • Total enrolled: 1165 participants at 39 sites (as of 9/30/12)
Protocol R: A Phase II Evaluation of Topical NSAIDs in Eyes with Non Central Involved DME Ø Objective • To assess the effects of topical NSAIDs on macular retina volume compared with placebo in eyes with non-central DME • To assess the effects of topical NSAIDs on central subfield thickness and to compare the progression of non-central DME to central DME as determined by OCT and stereoscopic fundus photographs 25
Protocol R continued Ø Ø Major Eligibility Criteria • Best corrected E-ETDRS VA letter score ≥ 74 (20/25 or better) • Definite retinal thickening due to DME within 3000 µm of the center of the macula but not involving the central subfield • No focal/grid laser within the last 6 months or other treatment for DME within the last 4 months Enrollment • Total enrolled: 125 subjects randomized at 40 sites (as of 10/7/13) 26

Protocol S: Prompt PRP versus Intravitreal Ranibizumab with Deferred PRP for PDR Ø Objective • To determine if visual acuity outcomes at 2 years in eyes with PDR that receive anti. VEGF therapy with deferred PRP are noninferior to those in eyes that receive standard prompt PRP therapy. 27

Protocol S: Prompt PRP versus Intravitreal Ranibizumab with Deferred PRP for PDR Ø Major Eligibility Criteria • Study eye with o o o Ø PDR for which PRP can be safely deferred for at least 4 weeks in the investigator’s judgment. No prior PRP Visual acuity letter score in the study eye > 24 (~ Snellen equivalent of 20/320 or better) Enrollment (Complete) • Total enrolled: 305 participants and 394 study eyes at 56 sites 28

Protocol T: A Comparative Effectiveness Study of Intravitreal Aflibercept, Bevacizumab and Ranibizumab for DME Ø Objective • To compare the efficacy and safety of intravitreal (1) aflibercept, (2) bevacizumab, and (3) ranibizumab when given to treat central-involved DME o Specifically, the primary outcome is to assess if either of these three anti-VEGF products is superior to the other with respect to mean changes in visual acuity. 29

Protocol T: A Comparative Effectiveness Study of Intravitreal Aflibercept, Bevacizumab and Ranibizumab for DME Ø Ø Major Eligibility Criteria • Study eye with o Central-involved DME (OCT CSF ≥ 250 µm on Zeiss Stratus or equivalent on spectral domain OCT). o Visual acuity letter score ≤ 78 and >24 (≈ Snellen 20/32 to 20/320) within eight days of randomization. o No prior intravitreal anti-VEG within prior 12 months Enrollment (complete) • Total enrolled: 660 subjects at 89 sites 30

Genes in Diabetic Retinopathy Project Ø • • Ø Ø Objective To create a repository of genetic material and clinical phenotype information as a resource for the research community The database may provide the opportunity to assess genetic susceptibility and resistance to DR and also variants impacting visually-important biomarkers for ME and neovascularization. Major Eligibility Criteria • Previous or current participant in a DRCR. net study Enrollment (Ongoing) 31 • Total enrolled: 807 subjects
Protocol V: Treatment for Central-Involved DME in Eyes with Very Good Visual Acuity Ø Objective: To compare the safety and efficacy of prompt focal/grid photocoagulation + deferred intravitreal anti-VEGF, observation + deferred intravitreal anti-VEGF, and prompt intravitreal anti -VEGF in eyes with central-involved DME and good visual acuity. • Good VA defined as a Snellen equivalent of 20/25 or better (electronic-ETDRS letter score of 79 or better). 32

Protocol V: Treatment for Central-Involved DME in Eyes with Very Good Visual Acuity Ø Major Eligibility Criteria: • Must be at two consecutive visits within 1 to 28 days: o o • • Ophthalmoscopic evidence of CI DME in study eye confirmed on OCT at two consecutive visits within 1 to 28 days; defined by OCT CSF thickness on Zeiss Cirrus or Heidelberg Spectralis OCT. VA letter score in study eye ≥ 79 (~Snellen equivalent 20/25 or better) at two consecutive visits within 1 to 28 days No history of prior laser or other surgical, intravitreal, or peribulbar treatment for DME in the study eye Enrollment Begins November 2013 33

Protocols In Development Ø Protocol U – Fall 2013 • Short-term Evaluation of Combination Corticosteroid+Anti-VEGF Treatment for Persistent Central-Involved Diabetic Macular Edema Following Anti-VEGF Therapy in Pseudophakic Eyes Ø New Protocols • Peripheral DR Lesions on Ultrawide-field Fundus Images and Risk of DR Worsening Over Time • Short-term Evaluation of Using a Different Anti-VEGF Drug Compared with Continuing on Bevacizumab in Eyes with Persistent Central-Involved Diabetic Macular Edema with Prior Bevacizumab Therapy 34

Thank you for attending! Your feedback is very important. Scanning this QR code will take you directly to the evaluation for this course. 186: Update on Treatments for Diabetic Retinopathy: Clinically Relevant Results from the Diabetic Retinopathy Clinical Research Network 35
- Slides: 35