The Cutter Incident Lessons from the Past Paul





























- Slides: 40

The Cutter Incident: Lessons from the Past Paul A. Offit Division of Infectious Diseases Children’s Hospital of Philadelphia University of Pennsylvania School of Medicine

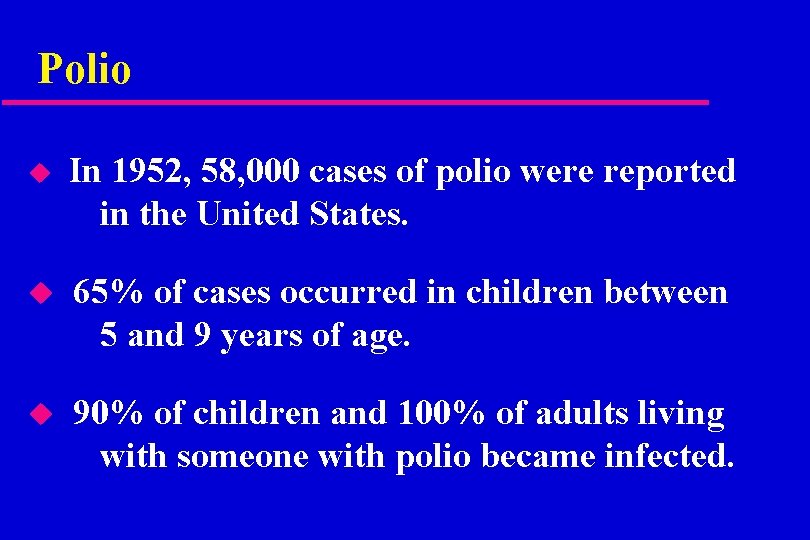
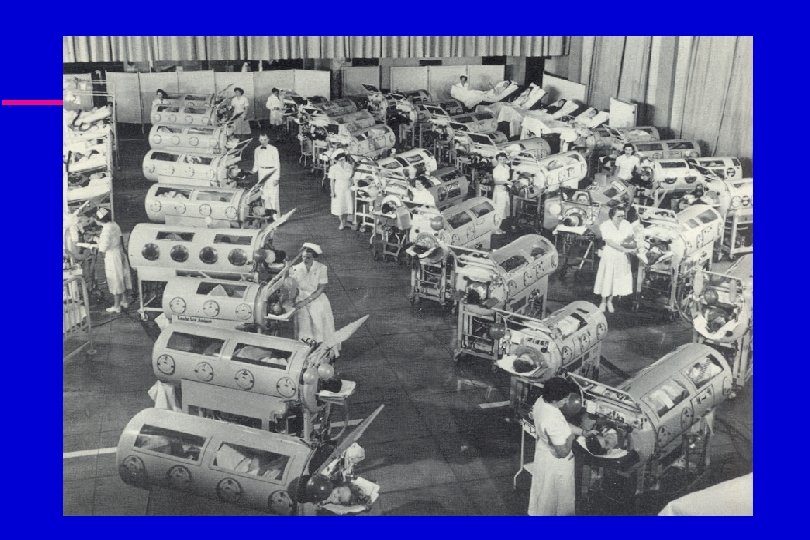
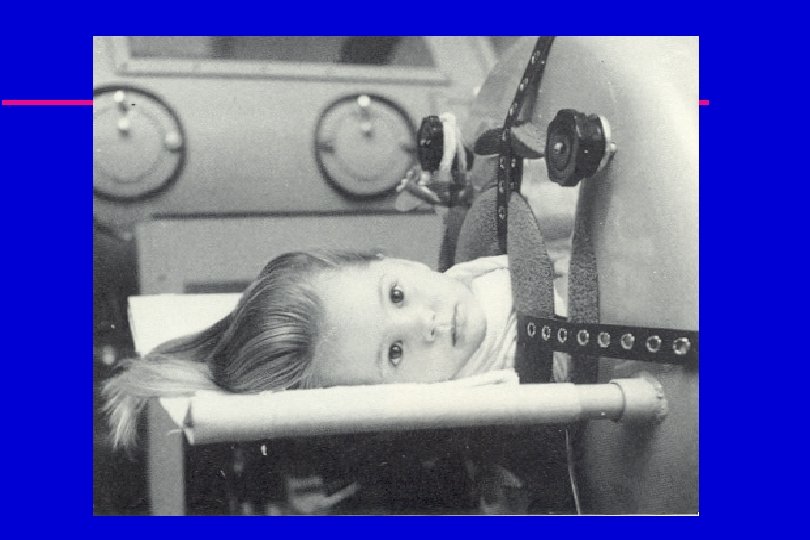
Polio u In 1952, 58, 000 cases of polio were reported in the United States. u 65% of cases occurred in children between 5 and 9 years of age. u 90% of children and 100% of adults living with someone with polio became infected.







The Salk vaccine u Three doses of vaccine given to 420, 000 children. u 200, 000 children inoculated with placebo and 1. 2 million observed, uninoculated. u Efficacy was 65% against type 1, 100% against type 2, and 96% against type 3 induced polio.


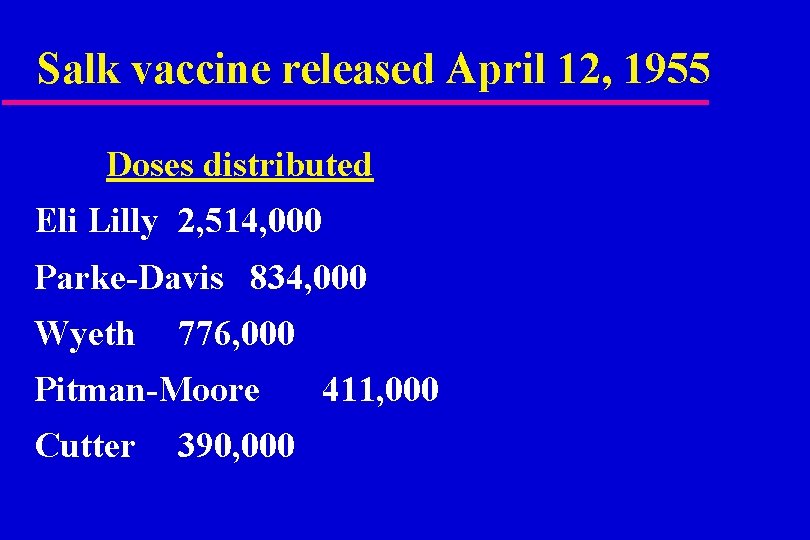
Salk vaccine released April 12, 1955 Doses distributed Eli Lilly 2, 514, 000 Parke-Davis 834, 000 Wyeth 776, 000 Pitman-Moore Cutter 390, 000 411, 000

The New York Times, April 28, 1955

Cutter made eight lots of vaccine Incidence of Doses paralysis/100, 000 19468 19764 121, 700 122 19460 19463 19467 19762 19766 19767 268, 900 15

Idaho u Vaccine made available free-of-charge from the National Foundation. u Idaho immunized 32, 000 school children (98% of first and second graders) in two weeks. u 20 children were paralyzed and 3 killed by Cutter’s vaccine.

Idaho u Examination of the medical records and interviews with parents of 425 children who received Cutter’s vaccine in Boise, Lewiston, and Pocatello. u 32. 7% had symptoms of abortive polio. Shaw, MB, et al. Am. J Dis Child 1958; 96: 58 -63.

The Cutter Incident u 120, 000 children inoculated with live poliovirus. u 100, 000 family and community contacts were also infected.

The Cutter Incident u At least 70, 000 developed abortive polio. u 164 people were permanently paralyzed. u 10 people were killed.




The Birth of Vaccine Regulation u Within one month of the Incident, the Laboratory of Biologics Control was changed to the Division of Biologics Standards. u The number of full-time, professional regulators increased from 10 to 150.

The Birth of the EIS u First real assignment of the Epidemic Intelligence Service and first national response to a medical emergency. u Gave tremendous credibility to the EIS and quickly garnered more funds for the agency.



Gottsdanker v. Cutter Laboratories u Negligence (lack of exercise of ordinary care). u Implied warranty (implied that a vaccine designed to prevent paralysis shouldn’t cause paralysis).

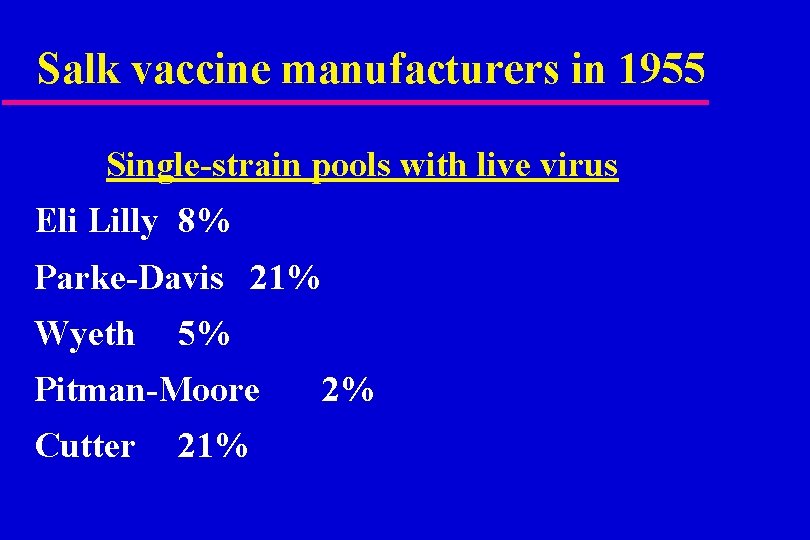
Salk vaccine manufacturers in 1955 Single-strain pools with live virus Eli Lilly 8% Parke-Davis 21% Wyeth 5% Pitman-Moore Cutter 21% 2%

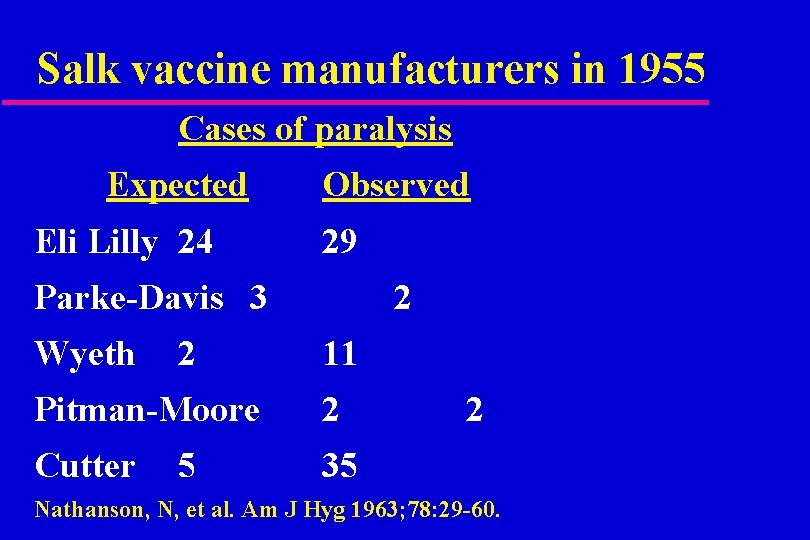
Salk vaccine manufacturers in 1955 Cases of paralysis Expected Eli Lilly 24 Observed 29 Parke-Davis 3 Wyeth 2 2 11 Pitman-Moore 2 Cutter 35 5 2 Nathanson, N, et al. Am J Hyg 1963; 78: 29 -60.


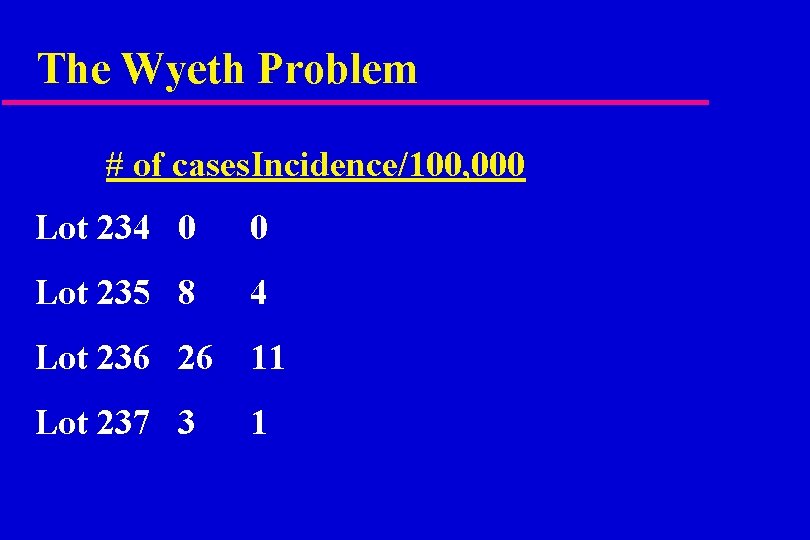
The Wyeth Problem # of cases. Incidence/100, 000 Lot 234 0 0 Lot 235 8 4 Lot 236 26 11 Lot 237 3 1

Gottsdanker v. Cutter Laboratories u Jury found Cutter not guilty of negligence but guilty of breech of implied warranty. Birth of liability without fault. u Why should Anne Gottsdanker have to buy insurance to protect her from paralysis caused by polio vaccine? u Company is in the best position to buy that insurance and distribute the cost.

Vaccines: 1955 -1975 u Difficult vaccines continued to be made, tested, and sold. u Measles and rubella vaccines each made after several relatively unsuccessful attempts.

The pertussis vaccine u Kulenkampff paper (1974) claimed that pertussis vaccine caused brain damage. u Hypothesis not confirmed by subsequent studies, but flood of lawsuits. u Price of DTP vaccine increased from $0. 17 to $11. 00 per dose. Number of companies making pertussis vaccine decreased from eight to one.

Toner v. Lederle Laboratories u In the mid-1980 s, parents of Kevin Toner claimed that the pertussis vaccine caused transverse myelitis. u Award against Lederle for $1. 13 million. u At the time of the verdict, the entire pertussis vaccine business in the U. S. was about $3 million.

Toner v. Lederle Laboratories Problems with liability without fault: u Without negligence, jury responsible for determining causality only (breast implants). u Jury determines the size of the awards (phen-phen).

NCVIA In 1986, the NCVIA was born: u Based on the vaccine injury table and funded by a federal excise tax on every vaccine (NVICP). u Model system to compensate those harmed by vaccines and protect vaccine makers from abuses by the tort system.

NVICP The NVICP is incomplete: u Plaintiffs can always opt out of the program if dissatisfied with the ruling. u Doesn’t cover all vaccines. u Doesn’t cover the unborn child when a pregnant woman is vaccinated.

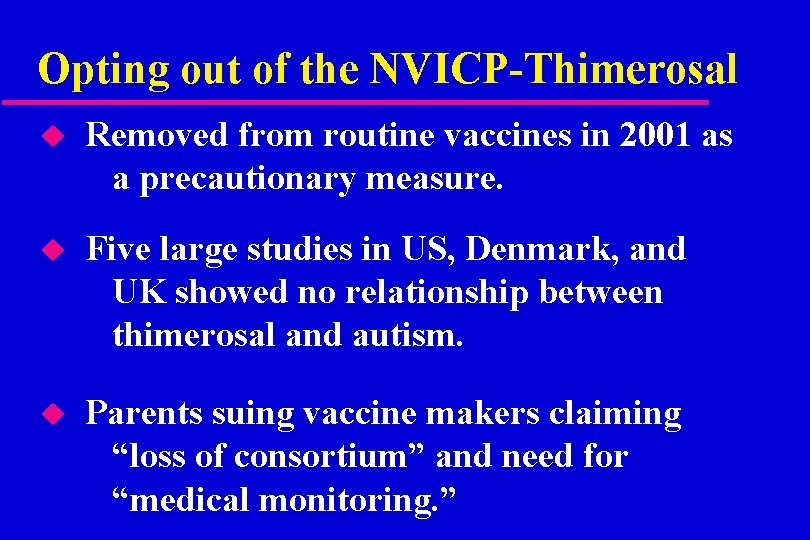
Opting out of the NVICP-Thimerosal u Removed from routine vaccines in 2001 as a precautionary measure. u Five large studies in US, Denmark, and UK showed no relationship between thimerosal and autism. u Parents suing vaccine makers claiming “loss of consortium” and need for “medical monitoring. ”

Incomplete coverage - Lyme vaccine u Made from outer surface protein (Osp. A). u Tested pre-licensure in 20, 000 people followed for two years. u Lawsuits filed successfully claiming vaccine caused chronic arthritis. u Publicity following those lawsuits in part caused vaccine to be discontinued.

The unborn child – GBS vaccine u Despite availability of antibiotic therapy, GBS causes 2, 000 hospitalizations and 100 deaths per year in US. u GBS-specific antibodies can be induced in pregnant women and passively transferred to newborns. u Vaccine makers would not dare to immunize pregnant women.

Vaccines for the future u Diseases like CMV, adenovirus, EBV cause hospitalization and death in select groups; may not be covered by VFC. u Technology is available, but willingness to make these vaccines isn’t?