The Corrosion Teachin Understanding the corrosion environment Different





















































- Slides: 110

The Corrosion Teach-in Understanding the corrosion environment

Different methods for corrosion control Coupons Online Monitors Inhibition programs Any method be made more effective…

…When you understand the effect of the corrosion environment Corrosion rates vary with process conditions

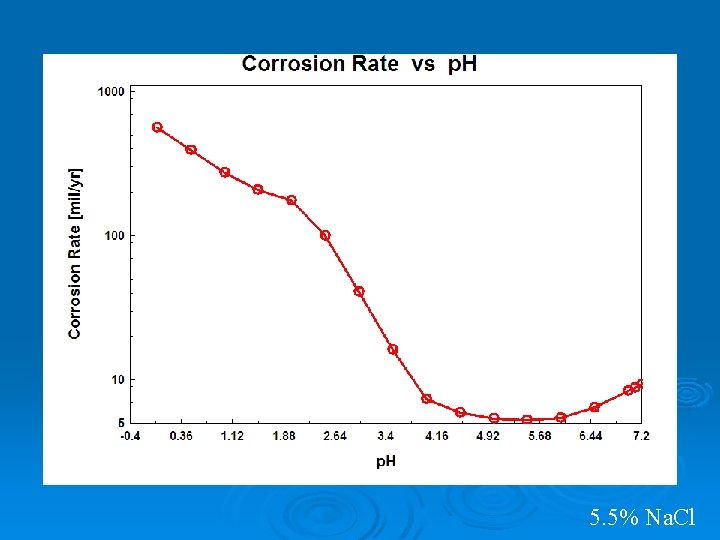
5. 5% Na. Cl

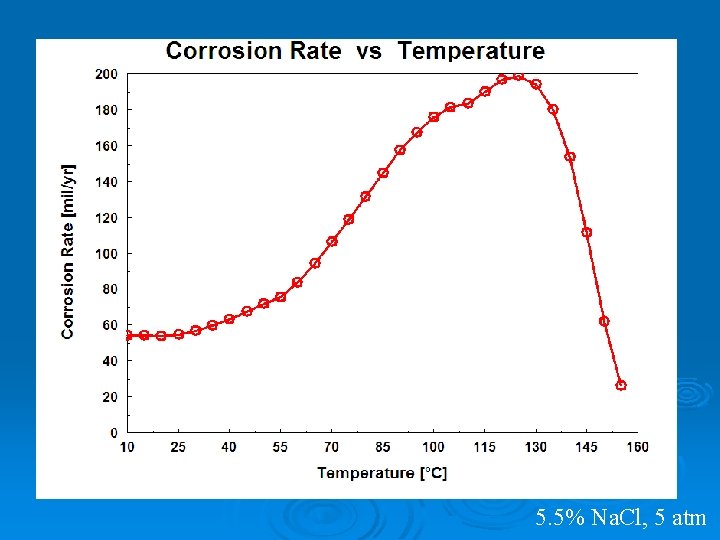
5. 5% Na. Cl, 5 atm

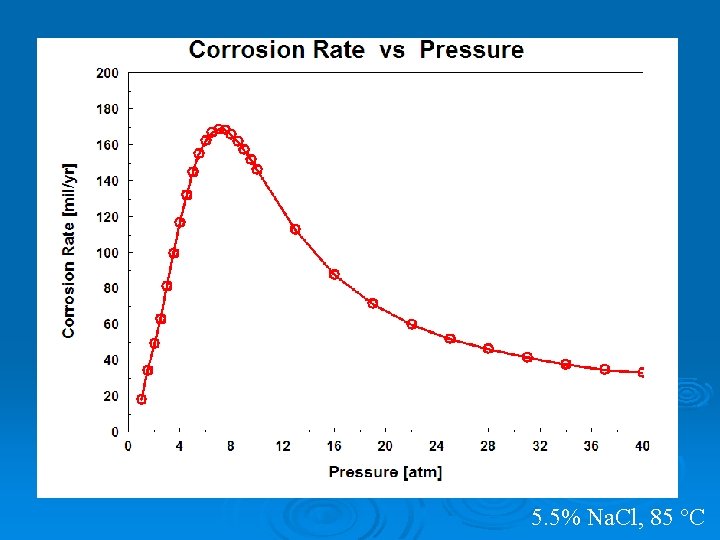
5. 5% Na. Cl, 85 °C

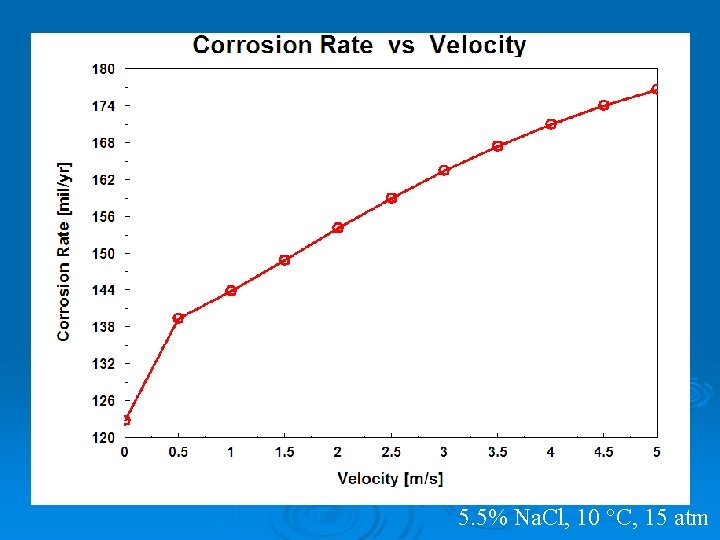
5. 5% Na. Cl, 10 °C, 15 atm

To interpret coupon and monitor data… It helps to know the effect of variations in the field

To locate where to place sensors & coupons… Wait for a failure…? Rely on past experience?

Coupons Online Monitors Tell you what has already happened, not what will happen

OLI tools can help OLI gets the chemistry right

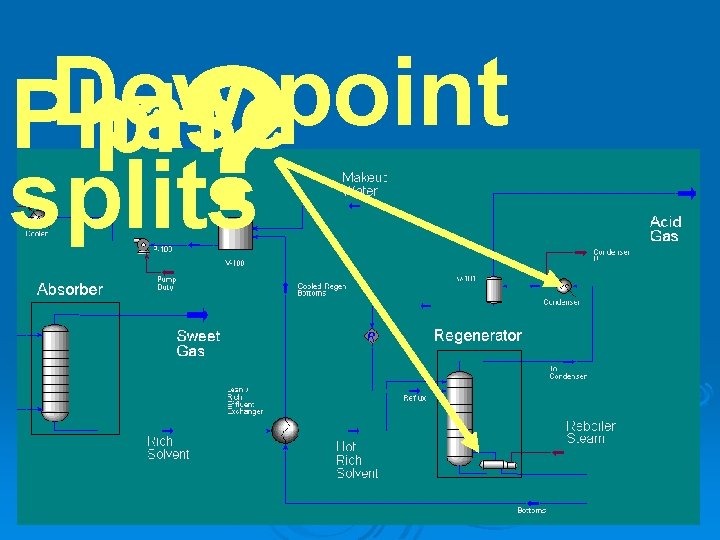
? Dew point Phase p. H splits

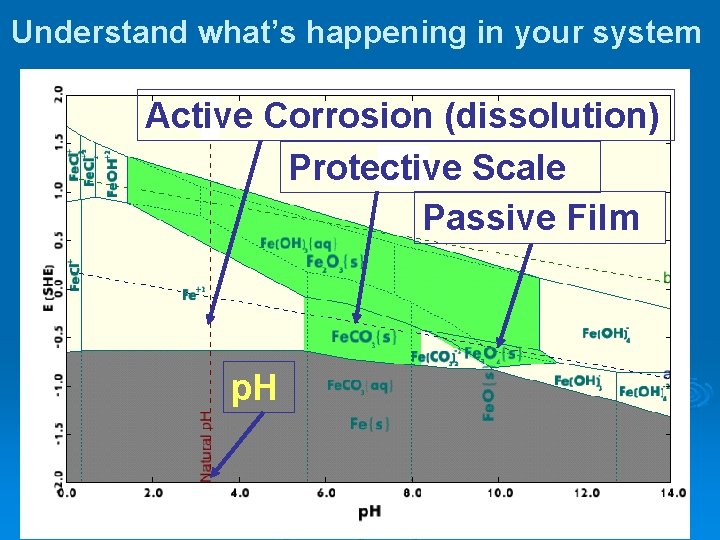
Understand what’s happening in your system Active Corrosion (dissolution) Protective Scale Passive Film p. H

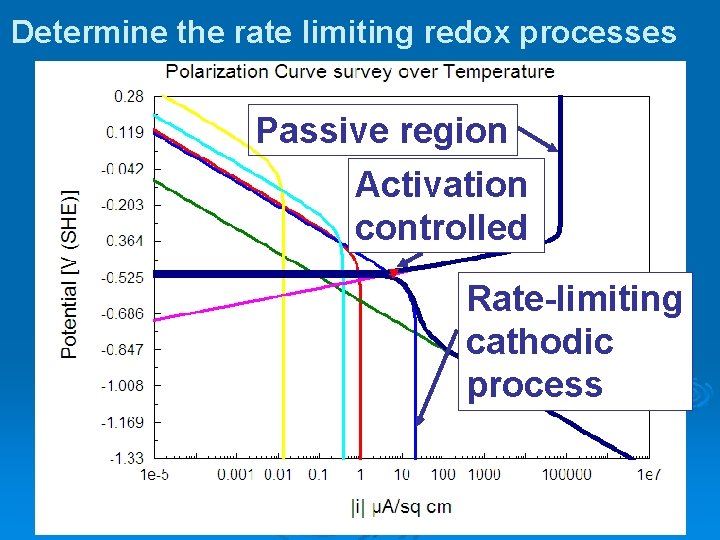
Determine the rate limiting redox processes Passive region Activation controlled Rate-limiting cathodic process

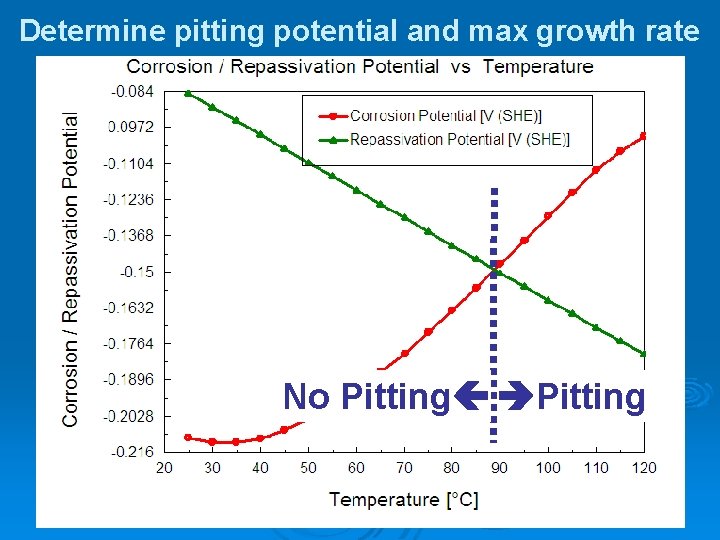
Determine pitting potential and max growth rate No Pitting

Pro-active Analysis ØTest Corrective Actions • Determine optimum p. H • Screen alloys and inhibitors • Assess process changes ØFocus Lab work ØEliminate potential problems before they occur

The Corrosion Analyzer Tool for understanding the corrosion environment Ø Mechanistically based software tool ØSpeciation ØKinetics of uniform corrosion Partial anodic and cathodic processes ØTransport properties ØRepassivation

The Corrosion Analyzer Based on the OLI Engine Ø Complete speciation model for complex mixtures § Phase and chemical reaction equilibria § Accurate p. H prediction § Redox chemistry § Comprehensive coverage of industrial chemical and petroleum systems

The Corrosion Analyzer Based on the OLI Engine Ø Thermophysical properties prediction Ø Phenomenological and unique aqueous process models including kinetics and transport Ø “Out of the box” solution and technical support

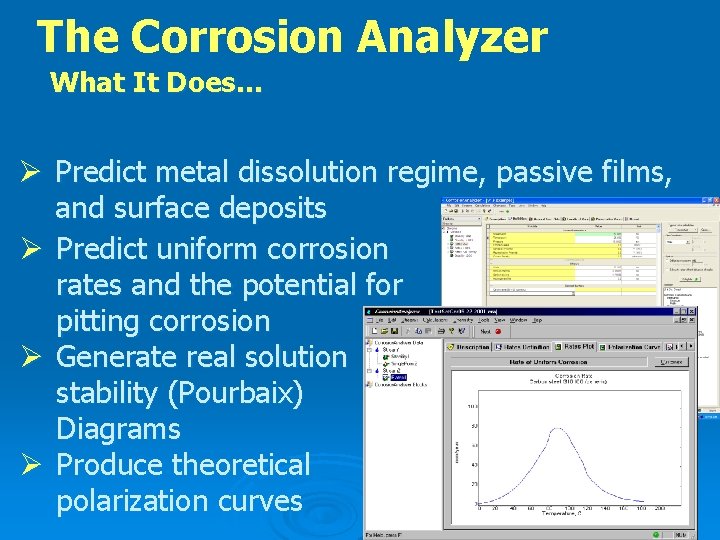
The Corrosion Analyzer What It Does… Ø Predict metal dissolution regime, passive films, and surface deposits Ø Predict uniform corrosion rates and the potential for pitting corrosion Ø Generate real solution stability (Pourbaix) Diagrams Ø Produce theoretical polarization curves

The Corrosion Analyzer So you can gain insight on … Corrosion mechanisms Rate-limiting partial processes for your operating conditions Ø Effects of process and materials changes Ø Ø Therefore Ø Focusing lab time Ø Reducing risky plant/field testing Ø Managing design, operation, and maintenance

Today’s seminar “Hands-on” and “How-To” ØUsing example problems ØExamining plots and diagrams ØUnderstanding the basis of the predictions

Today’s Seminar Ø Ø Ø Perform “Single point” calculations Construct / interpret real solution Pourbaix Diagrams Calculate corrosion rates ØEvaluate the effects of p. H, T, comp / flow Ø Evaluate polarization curves ØGain insight to corrosion mechanisms ØSee rate limiting steps ØCan I read them? Can I trust them? Ø Determine the likelihood of pitting to occur For your actual field or lab conditions

Welcome to the CORROSION TEACH-IN Simulating Real World Corrosion Problems

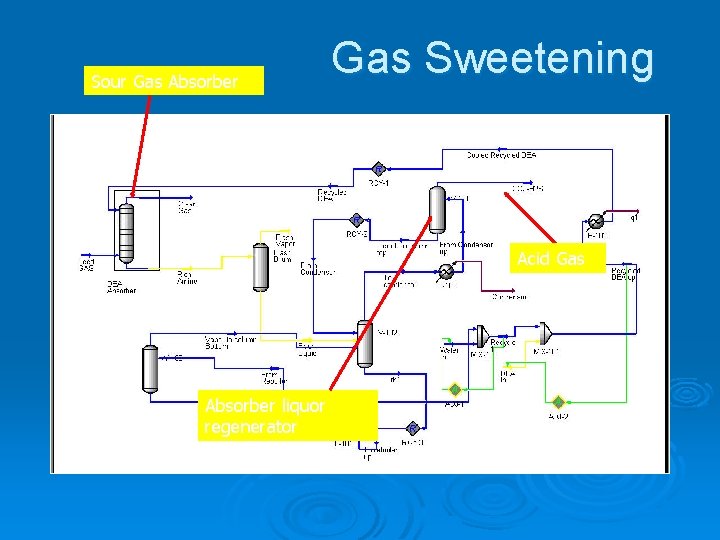
Gas Condensate Corrosion Ø Scope l Gas condensates from alkanolamine gas sweetening plants can be highly corrosive. Ø Purpose l Diethanolamine is used to neutralize (sweeten) a natural gas stream. This removes carbon dioxide and hydrogen sulfide. The off gas from the regeneration is highly acidic and corrosive

Gas Condensate Corrosion Ø Objectives l l l Determine the dew point of the acid gas Remove the condensed phase and perform corrosion rate calculations Mitigate the corrosion

Sour Gas Absorber Gas Sweetening Acid Gas Absorber liquor regenerator

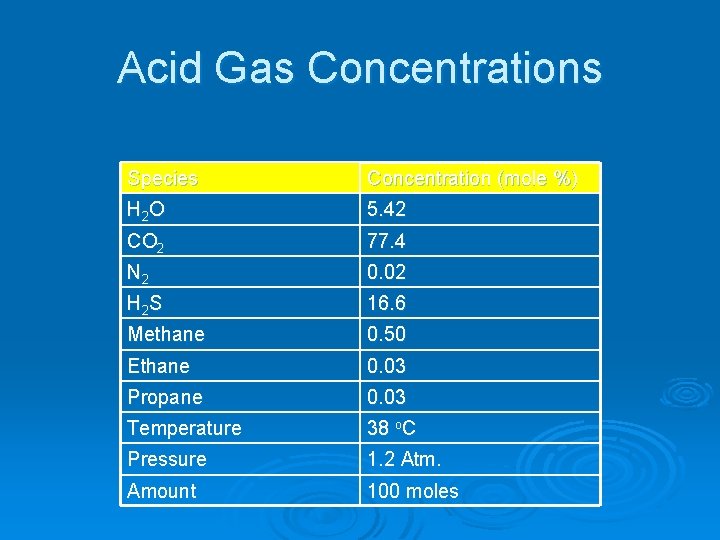
Acid Gas Concentrations Species Concentration (mole %) H 2 O 5. 42 CO 2 77. 4 N 2 0. 02 H 2 S 16. 6 Methane 0. 50 Ethane 0. 03 Propane 0. 03 Temperature 38 o. C Pressure 1. 2 Atm. Amount 100 moles

Application Time

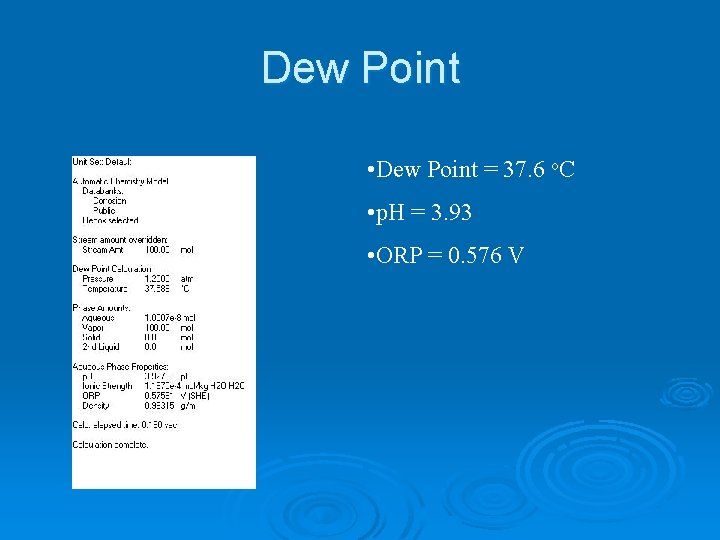
Dew Point • Dew Point = 37. 6 o. C • p. H = 3. 93 • ORP = 0. 576 V

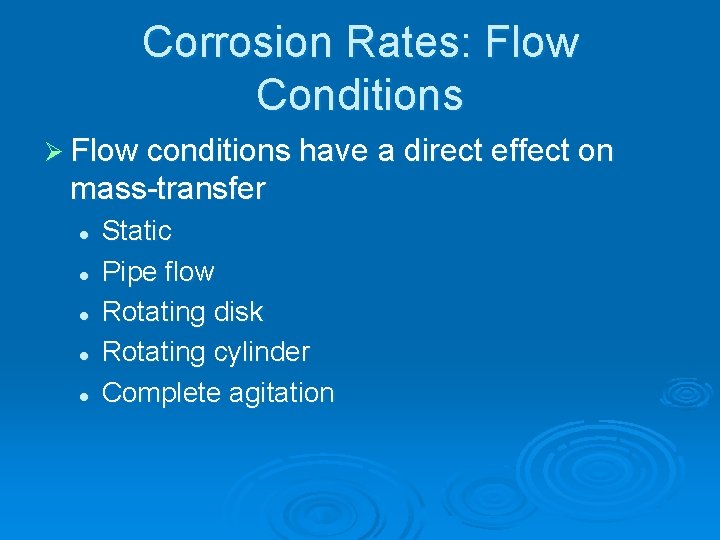
Corrosion Rates: Flow Conditions Ø Flow conditions have a direct effect on mass transfer l l l Static Pipe flow Rotating disk Rotating cylinder Complete agitation

Application Time

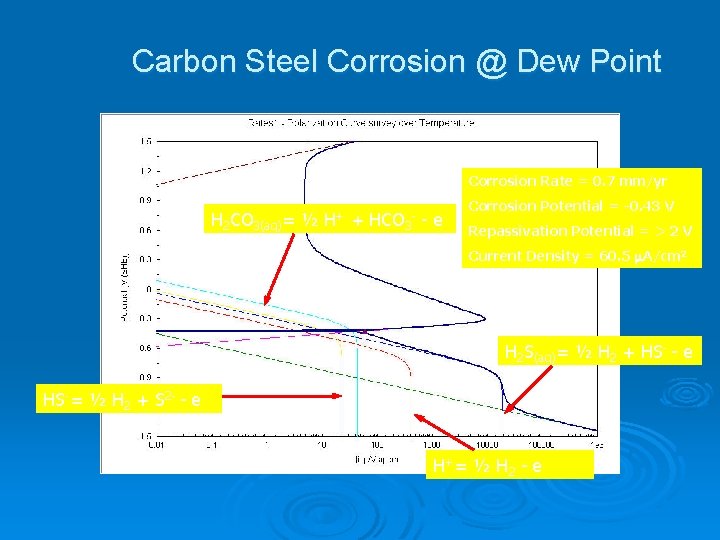
Carbon Steel Corrosion @ Dew Point Corrosion Rate = 0. 7 mm/yr H 2 CO 3(aq)= ½ H+ + HCO 3 - e - Corrosion Potential = -0. 43 V Repassivation Potential = > 2 V Current Density = 60. 5 A/cm 2 H 2 S(aq)= ½ H 2 + HS- - e HS-= ½ H 2 + S 2 - - e H+= ½ H 2 - e

Mitigation Ø Adjusting solution chemistry Ø Temperature profiling Ø Alloy screening Ø Cathodic protection

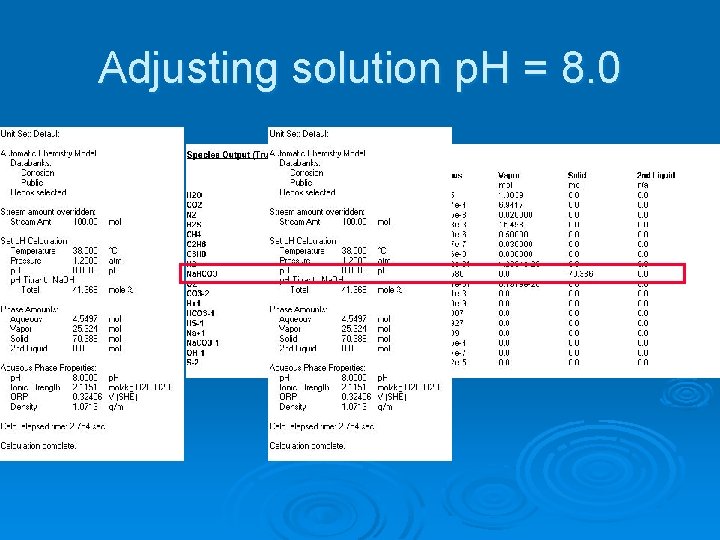
Adjusting the Solution Chemistry Ø Changing operating p. H l Add acid or base

Application Time

Adjusting solution p. H = 8. 0

Screening Alloys Ø Select an alloy that has a preferential corrosion rate l l 13% chromium 304 Stainless

Application Time

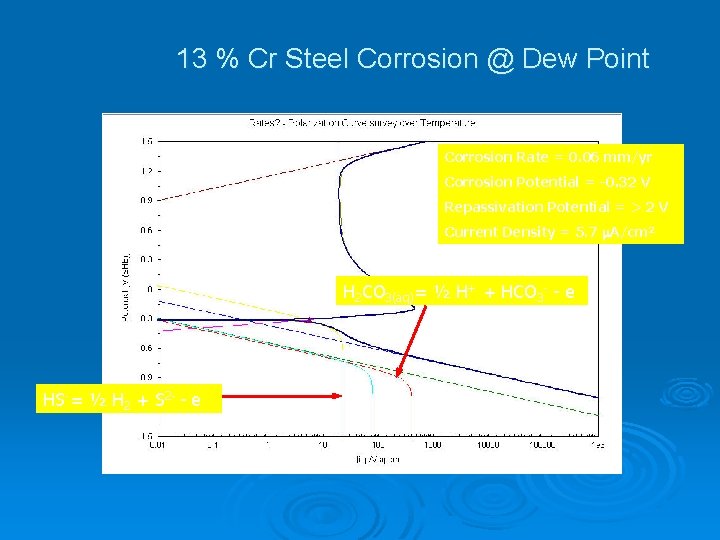
13 % Cr Steel Corrosion @ Dew Point Corrosion Rate = 0. 06 mm/yr Corrosion Potential = -0. 32 V Repassivation Potential = > 2 V Current Density = 5. 7 A/cm 2 H 2 CO 3(aq)= ½ H+ + HCO 3 - - e HS-= ½ H 2 + S 2 - - e

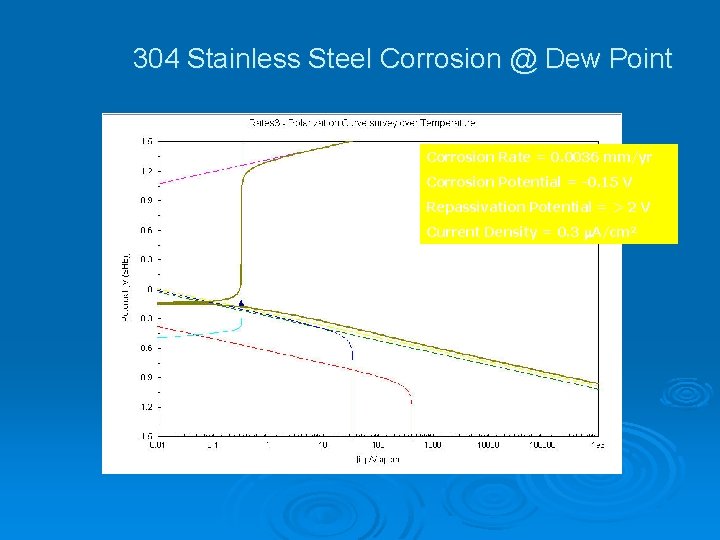
304 Stainless Steel Corrosion @ Dew Point Corrosion Rate = 0. 0036 mm/yr Corrosion Potential = -0. 15 V Repassivation Potential = > 2 V Current Density = 0. 3 A/cm 2

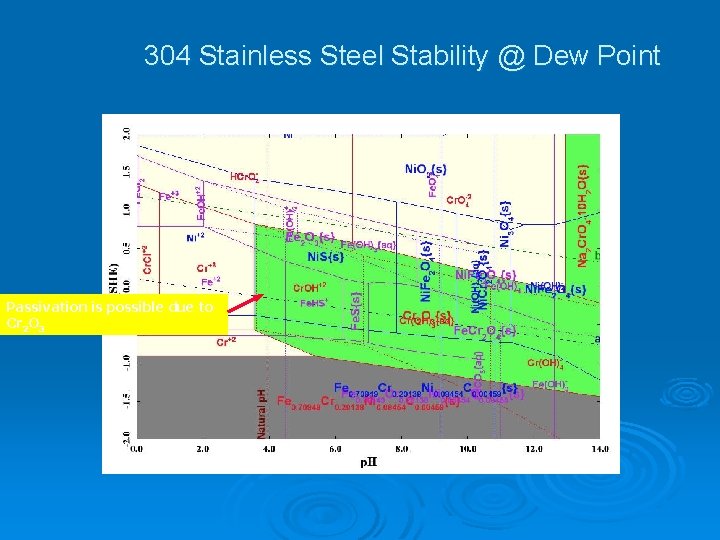
304 Stainless Steel Stability @ Dew Point Passivation is possible due to Cr 2 O 3

Why Iron Rusts Explaining common observations using Stability Diagrams

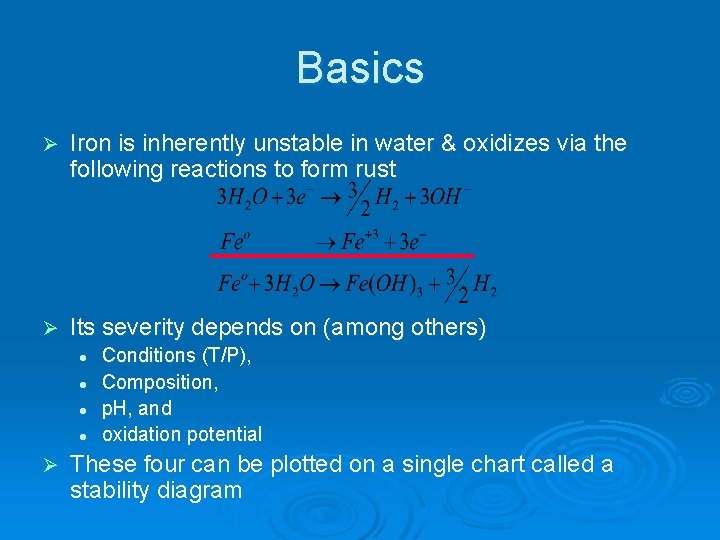
Basics Ø Iron is inherently unstable in water & oxidizes via the following reactions to form rust Ø Its severity depends on (among others) l l Ø Conditions (T/P), Composition, p. H, and oxidation potential These four can be plotted on a single chart called a stability diagram

Start example

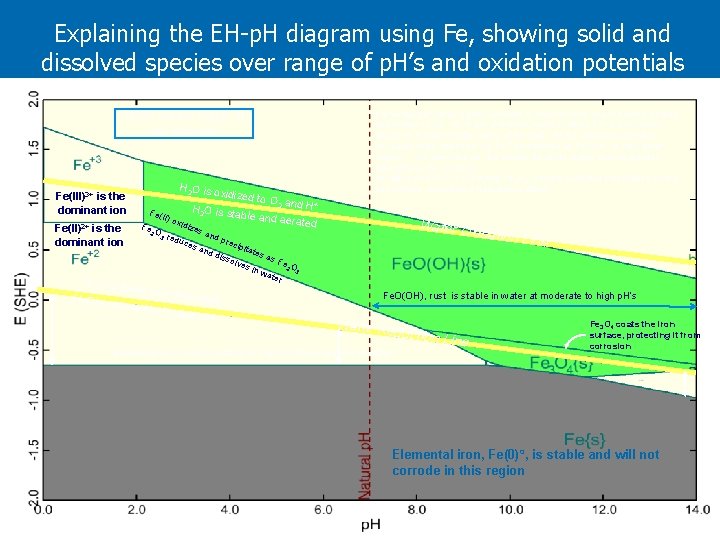
Explaining the EH-p. H diagram using Fe, showing solid and dissolved species over range of p. H’s and oxidation potentials White area is region of iron corrosion Fe(III)3+ is the dominant ion Fe(II)2+ is the dominant ion H 2 O is s H 2 O is o xidized to O 2 and H + H 2 O is sta Fe( ble and a II) o erated xid Fe 2 O 3 Water Ox izes and red pre uce cipi s an tate d di s as sso Fe lves 2 O in w 3 ater table and deaerate d duced to H 2 and O H H 2 O is re Elemental iron (gray region) corrodes in water to form one of several phases, depending on p. H. At ~9 p. H and lower, water oxidizes Fe 0 to Fe+2 which dissolves in water (white region of the plot). As the oxidation potential increases (high dissolved O 2) Fe+2 precipitates as Fe. OOH, or rust (green region). The lower the p. H, the thicker the white region and the greater driving force for corrosion At higher p. H (10 11), Fe 0 forms Fe 3 O 4, a stable solid that precipitates on the iron surface, protecting it from further attack. idation Lin e Fe. O(OH), rust is stable in water at moderate to high p. H’s Water Re duction L Elemental iron, Fe(0) oxidizes to Fe(II) in the presence of water ine Fe 3 O 4 coats the iron surface, protecting it from corrosion Elemental iron, Fe(0) o, is stable and will not corrode in this region

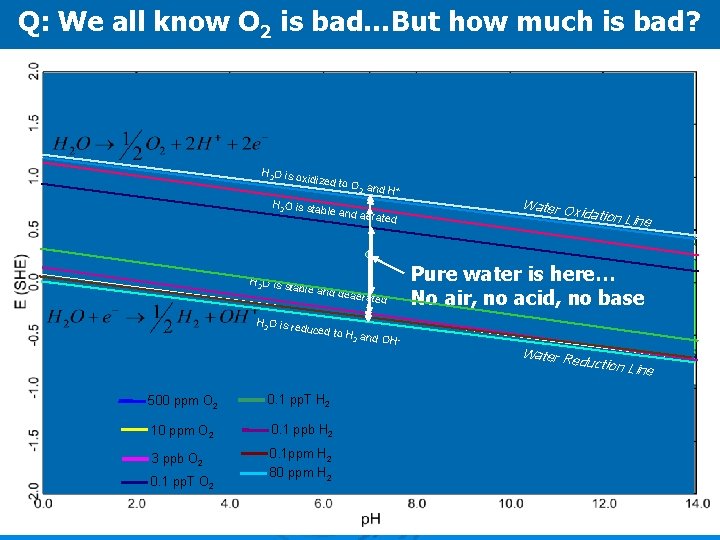
Q: We all know O 2 is bad…But how much is bad? H 2 O is o xidized to H 2 O is sta O 2 and H + ble and a H 2 O is s table and H 2 O is re erated deaerate duced to d H 2 and O H Water Ox idation Lin e Pure water is here… No air, no acid, no base Water Re duction L ine 500 ppm O 2 0. 1 pp. T H 2 10 ppm O 2 0. 1 ppb H 2 3 ppb O 2 0. 1 ppm H 2 80 ppm H 2 0. 1 pp. T O 2

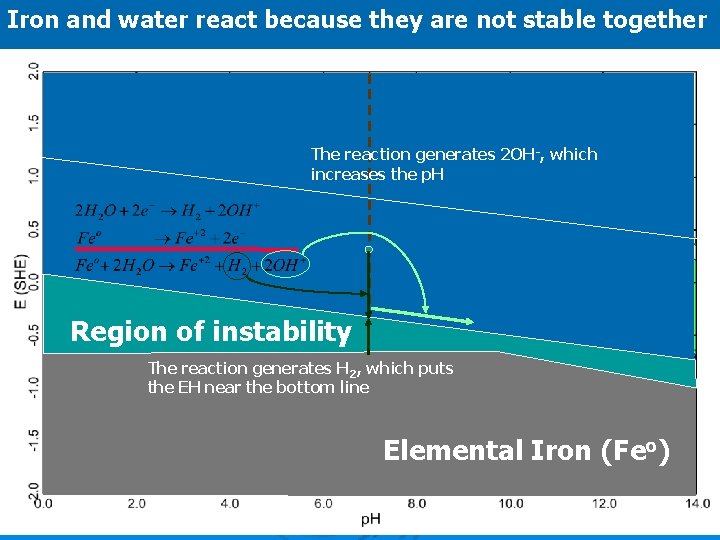
Iron and water react because they are not stable together The reaction generates 2 OH-, which increases the p. H Region of instability The reaction generates H 2, which puts the EH near the bottom line Elemental Iron (Feo)

Why is Stainless Steel stainless?

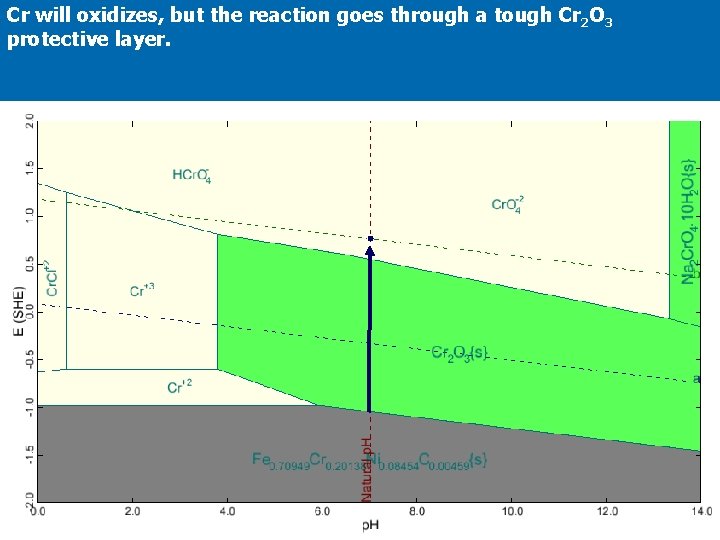
Cr will oxidizes, but the reaction goes through a tough Cr 2 O 3 protective layer.

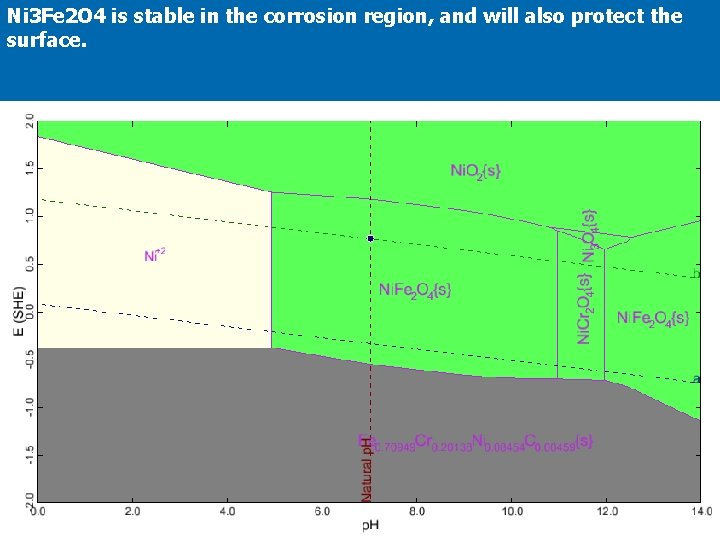
Ni 3 Fe 2 O 4 is stable in the corrosion region, and will also protect the surface.

Welcome to the CORROSION TEACH-IN Simulating Real World Corrosion Problems

Corrosion in Seawater Ø Scope l l Metals used for handling sea water face both general and localized corrosion. Various grades of stainless steels have been used to mitigate the problems. Stainless steels owe their corrosion resistance to a thin adherent film of oxides on their surface. Disruption of the films can lead to localized corrosion and premature failure.

Corrosion in Seawater Ø Purpose l l Chlorine and oxygen in sea water can attack the films used to passivate the steels. The Corrosion. Analyzer will be used to model the effects of chloride and oxygen on the rates of uniform corrosion and the possibility of pitting on the surface of the metals.

Corrosion in Seawater Ø Objectives l l l Reconcile a sea water sample for electroneutrality Reconcile a gas analysis Calculate uniform rates of corrosion for • • • 304 stainless steel 316 stainless steel S 31254 stainless steel

Corrosion in Seawater Ø Objectives (continued) l Determine the probability of pitting using the localized corrosion feature.

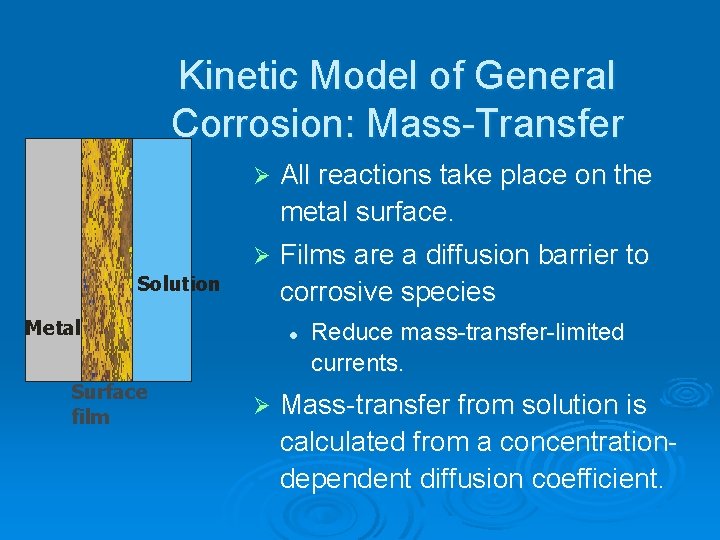
Kinetic Model of General Corrosion: Mass Transfer Ø All reactions take place on the metal surface. Ø Films are a diffusion barrier to corrosive species Solution Metal Surface film l Ø Reduce mass transfer limited currents. Mass transfer from solution is calculated from a concentration dependent diffusion coefficient.

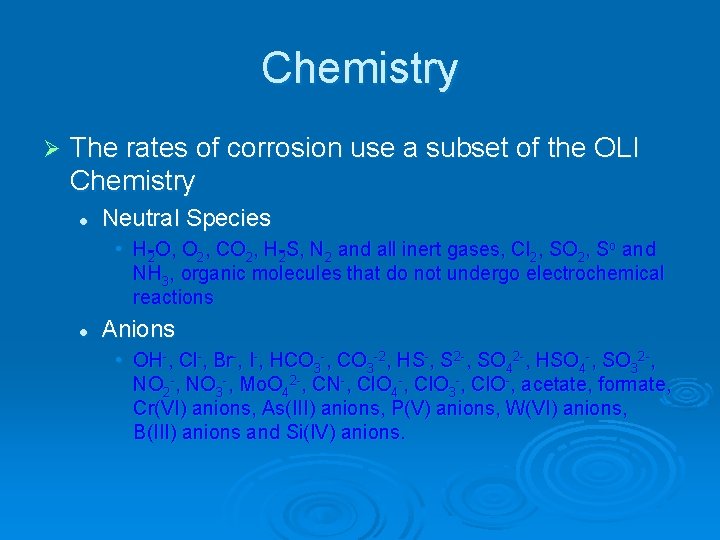
Chemistry Ø The rates of corrosion use a subset of the OLI Chemistry l Neutral Species • H 2 O, O 2, CO 2, H 2 S, N 2 and all inert gases, Cl 2, SO 2, So and NH 3, organic molecules that do not undergo electrochemical reactions l Anions • OH , Cl , Br , I , HCO 3 , CO 3 2, HS , S 2 , SO 42 , HSO 4 , SO 32 , NO 3 , Mo. O 42 , CN , Cl. O 4 , Cl. O 3 , Cl. O , acetate, formate, Cr(VI) anions, As(III) anions, P(V) anions, W(VI) anions, B(III) anions and Si(IV) anions.

Chemistry l Cations • H+, alkali metals, alkaline earth metals, Fe(II) cations, Fe(III) cations, Al(III) cations, Cd(II) cations, Sn(II) cations, Zn(II) cations, Cu(II) cations, Pb(II) cations and NH 4+.

Corrosion of 304 Stainless Steel in Deaerated Sea Water Species Concentrati on (mg/L) Cl 19000 Na+ 10700 Mg+2 1300 Ca+2 400 SO 4 2 2750 HCO 3 150 p. H 8. 0 Temperatu 25 o. C re Pressure 1 atm. Ø Lab. Analyzer used to reconcile electroneutrality Ø Na. OH/HCl Used to adjust p. H

Application Time

Screening Considerations Ø Some alloys do not perform well in seawater Ø We will evaluate 3 stainless steels l l Uniform corrosion rates Pitting possibility Ø Considering both deaerated and aerated conditions

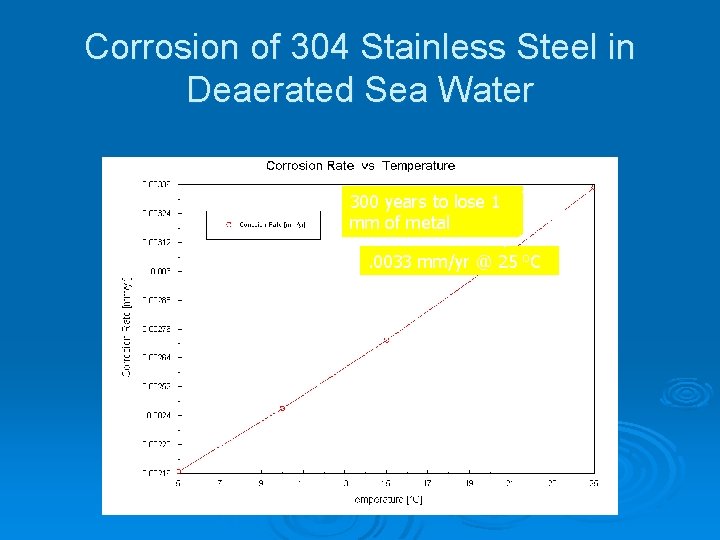
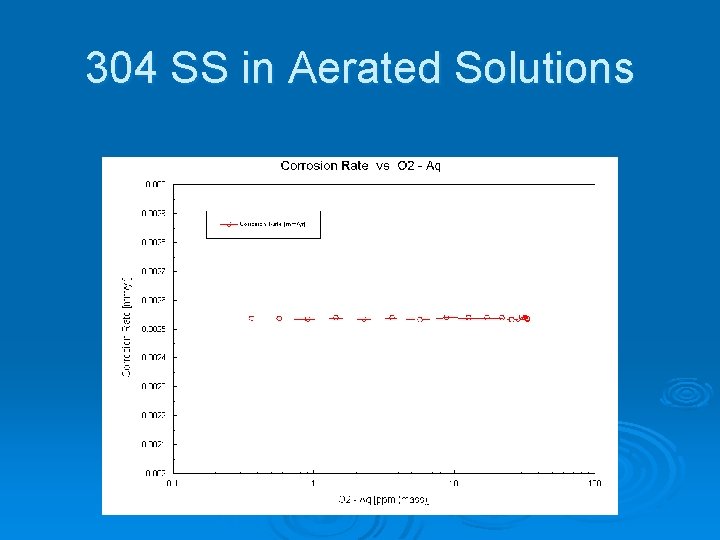
Corrosion of 304 Stainless Steel in Deaerated Sea Water 300 years to lose 1 mm of metal. 0033 mm/yr @ 25 o. C

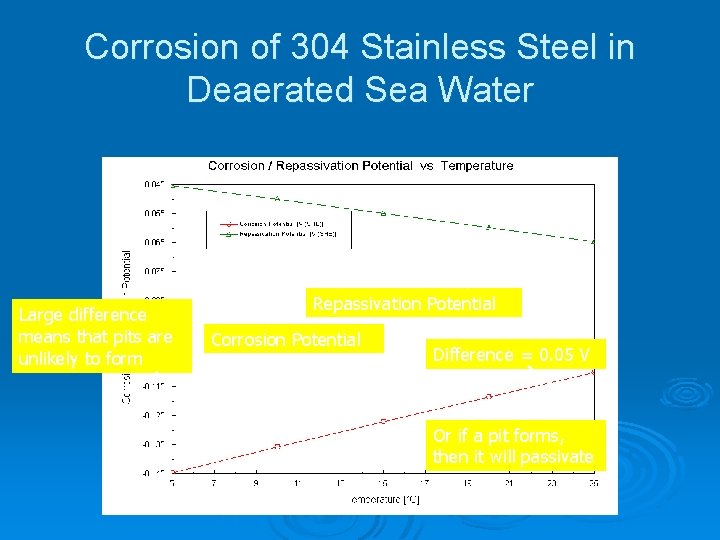
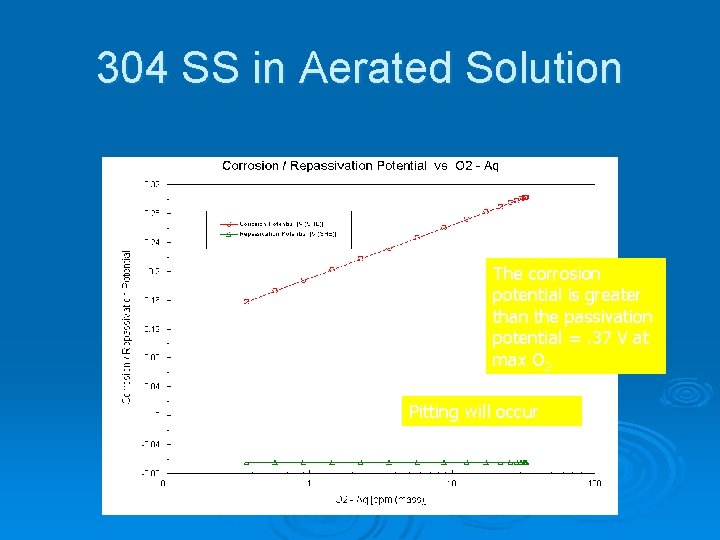
Corrosion of 304 Stainless Steel in Deaerated Sea Water Large difference means that pits are unlikely to form Repassivation Potential Corrosion Potential Difference = 0. 05 V Or if a pit forms, then it will passivate

Application Time

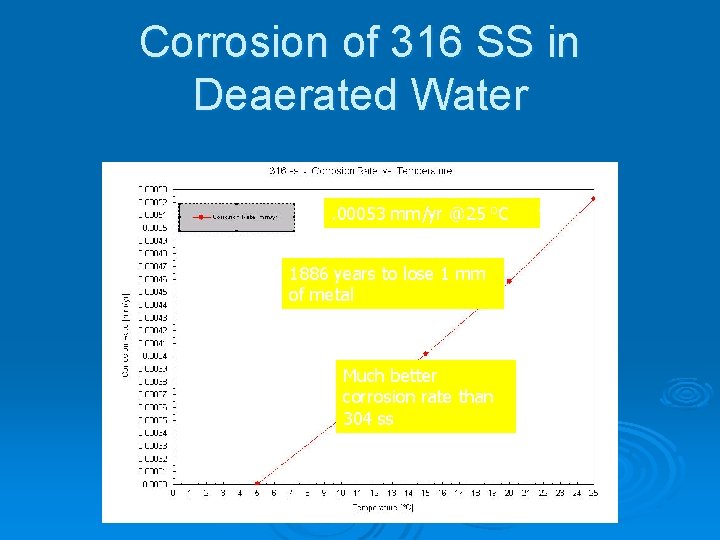
Corrosion of 316 SS in Deaerated Water. 00053 mm/yr @25 o. C 1886 years to lose 1 mm of metal Much better corrosion rate than 304 ss

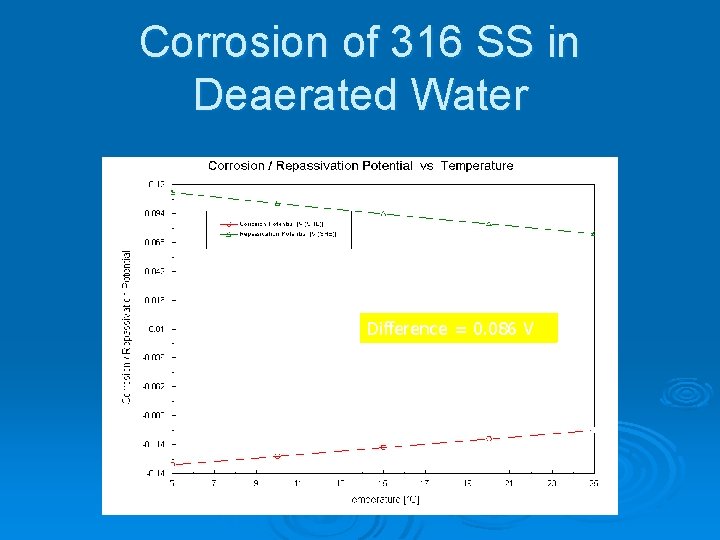
Corrosion of 316 SS in Deaerated Water Difference = 0. 086 V

Application Time

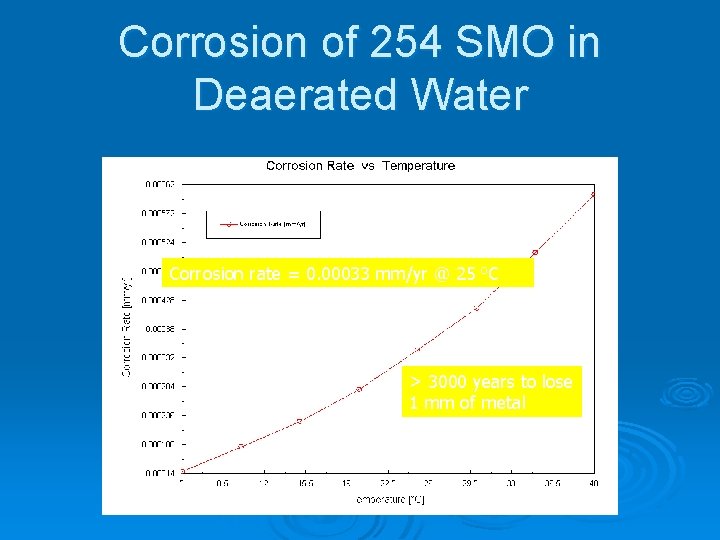
Corrosion of 254 SMO in Deaerated Water Corrosion rate = 0. 00033 mm/yr @ 25 o. C > 3000 years to lose 1 mm of metal

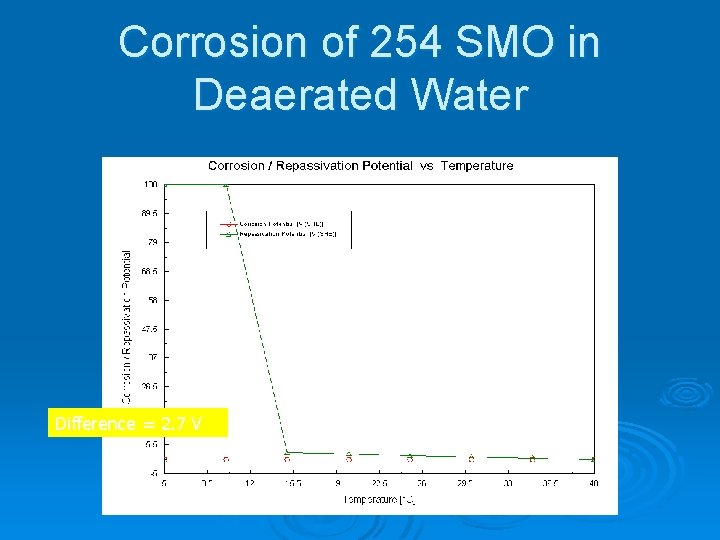
Corrosion of 254 SMO in Deaerated Water Difference = 2. 7 V

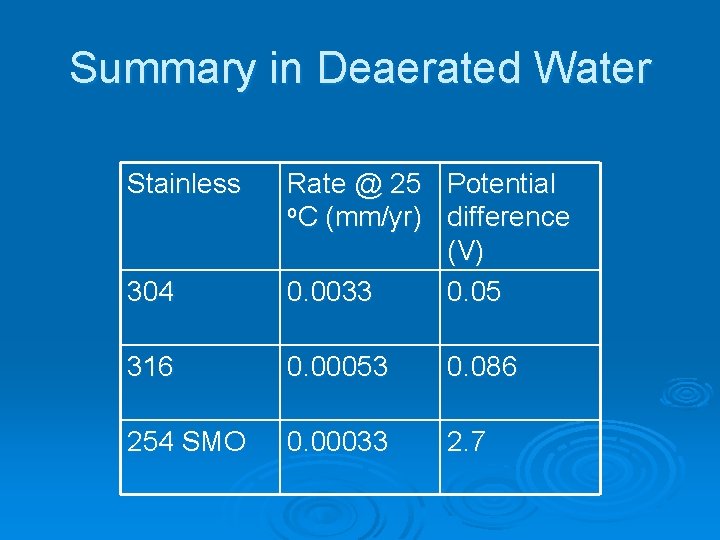
Summary in Deaerated Water Stainless 304 Rate @ 25 Potential o. C (mm/yr) difference (V) 0. 0033 0. 05 316 0. 00053 0. 086 254 SMO 0. 00033 2. 7

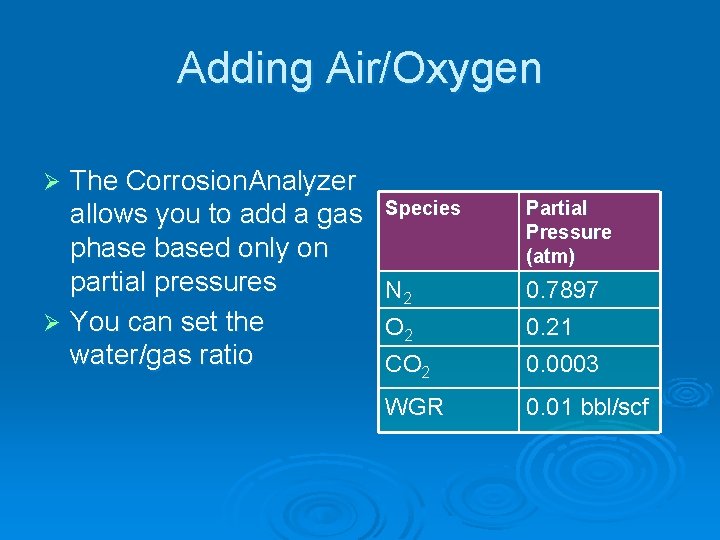
Adding Air/Oxygen The Corrosion. Analyzer allows you to add a gas phase based only on partial pressures Ø You can set the water/gas ratio Ø Species Partial Pressure (atm) N 2 0. 7897 O 2 0. 21 CO 2 0. 0003 WGR 0. 01 bbl/scf

Application Time

304 SS in Aerated Solutions

304 SS in Aerated Solution The corrosion potential is greater than the passivation potential =. 37 V at max O 2 Pitting will occur

Application Time

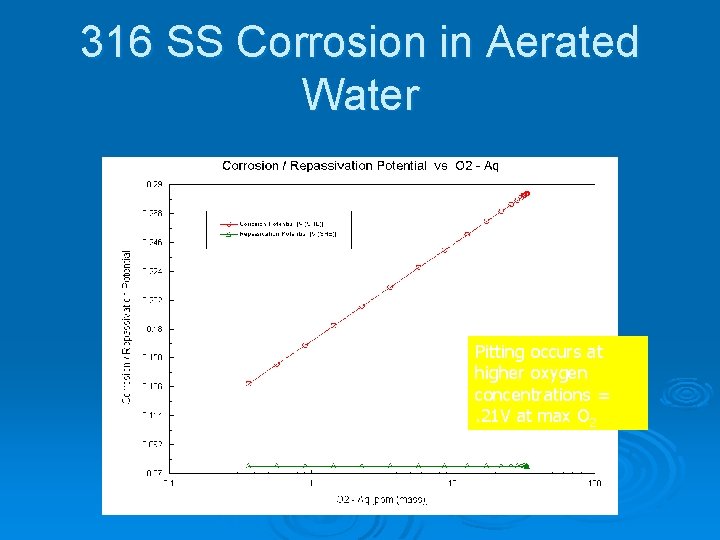
316 SS Corrosion in Aerated Water Pitting occurs at higher oxygen concentrations =. 21 V at max O 2

Application Time

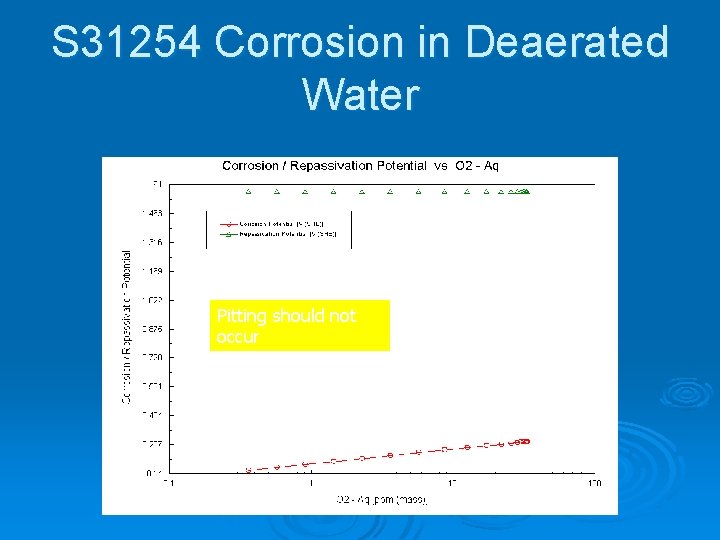
S 31254 Corrosion in Deaerated Water Pitting should not occur

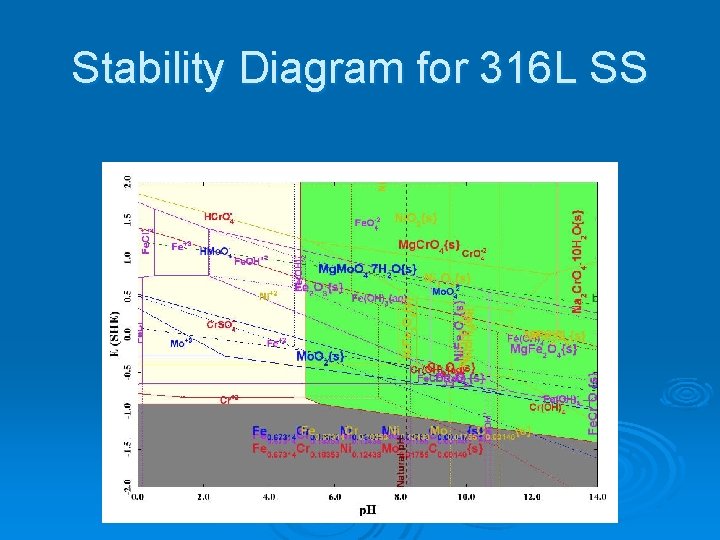
Stability Diagram for 316 L SS

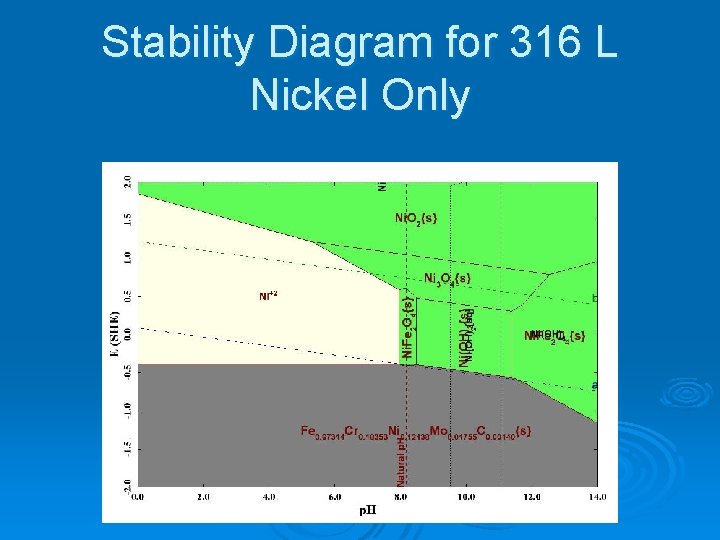
Stability Diagram for 316 L Nickel Only

Mitigation Ø Change Alloys l l Ø S 31254 seems the best at 25 o. C S 31254 increased potential for pitting at higher temperatures Cathodic Protection l l l Shifting of potential to less corrosive potentials via a sacrificial anode. Analyzers do not model CP Polarization curves can help determine the change in potential.

Welcome to the CORROSION TEACH-IN Simulating Real World Corrosion Problems

Dealloying of Copper Nickel Alloys Ø Scope l A copper nickel pipe made of Cupronickel 30 has been preferentially dealloyed while in contact with a 26 weight percent calcium chloride brine. It appears that the nickel in the alloy has been preferentially removed.

Dealloying of Copper Nickel Alloys Ø Purpose l The OLI/Corrosion. Analyzer will be used to show the relative stability of nickel and copper in the cupronickel alloy in an aqueous solution. It will show that protective films were not present as originally thought.

Dealloying of Copper Nickel Alloys Ø Objectives l l l Input information into the software and perform calculations Use stability diagrams to display information about the alloy and the protective films Change the diagrams to view different aspects of the stability of the alloy

Application: Dealloying of Copper Nickel Alloys A cupronickel 30 pipe (30 mass % copper) was used. Ø 26 wt % Ca. Cl 2 solution was in contact with the pipe. Ø Nickel was preferentially removed. Ø Dealloyed cupronickel pipe.

Questions? Ø Why did the nickel dealloy from the pipe? Ø What could we do to prevent this from occurring? Ø Which tools are available to understand this phenomenon?

Which Tools are Available? Ø A Pourbaix diagram can help us determine where metals are stable. l Corrosion. Analyzer

Creating the First Stability Diagram We will use the Corrosion. Analyzer to create a stability diagram for this system. Ø Features of Corrosion. Analyzer diagrams Ø l l Real solution activity coefficients Elevated temperatures Elevated pressures Interactions between species and overlay of diagrams.

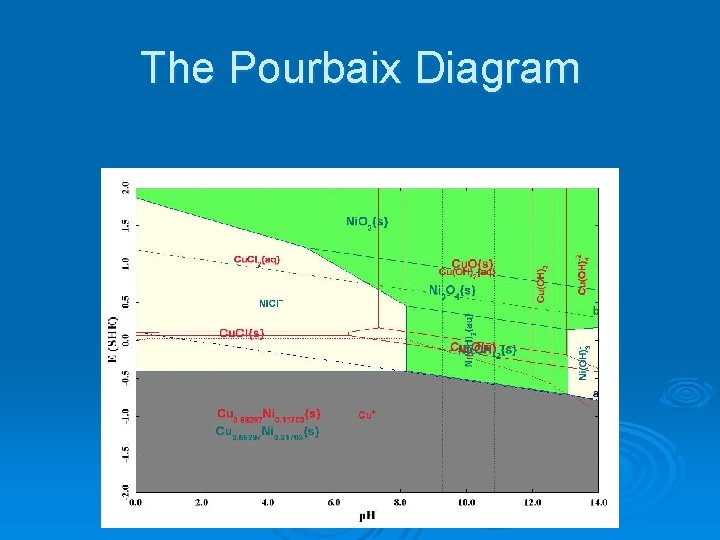
The Pourbaix Diagram

Application Time to start working with the OLI Corrosion Analyzer

The Pourbaix Diagram Ø There are quite a few things to look at on this diagram. l l Ø Stability field for water Stability fields for nickel metal and copper metal Stability fields for nickel and copper oxides Stability fields for aqueous species. We will now break down the diagram in to more manageable parts.

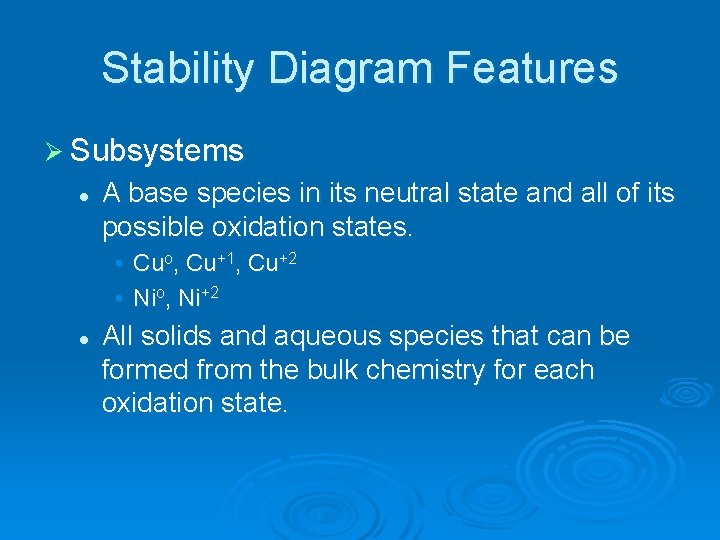
Stability Diagram Features Ø Subsystems l A base species in its neutral state and all of its possible oxidation states. • Cuo, Cu+1, Cu+2 • Nio, Ni+2 l All solids and aqueous species that can be formed from the bulk chemistry for each oxidation state.

Stability Diagram Features Ø For each subsystem l Contact Surface • Base metals • Alloys l Films • Solids l l Solid Lines Aqueous Lines

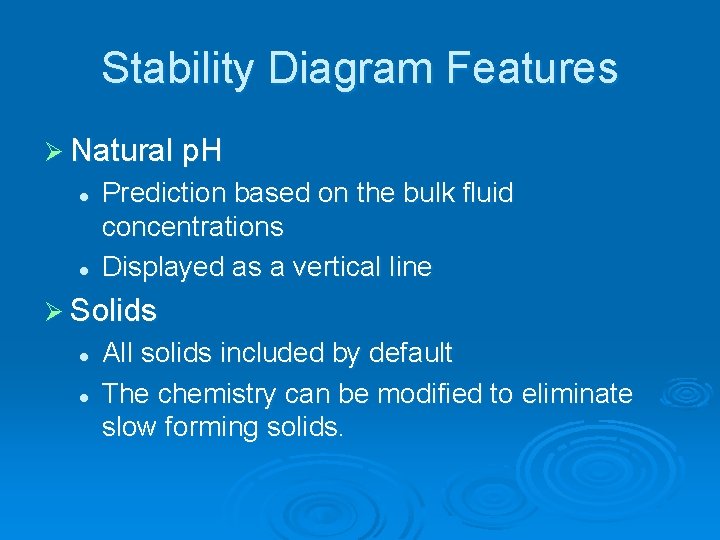
Stability Diagram Features Ø Natural p. H l l Prediction based on the bulk fluid concentrations Displayed as a vertical line Ø Solids l l All solids included by default The chemistry can be modified to eliminate slow forming solids.

Stability Diagram Features Ø Passivity l l l Thin, oxidized protective films forming on metal or alloy surfaces. Transport barrier of corrosive species to metal surface. Blocks reaction sites

Water Stability Ø Water can act as an oxidizing agent l Water is reduced to hydrogen, H 2 Ø Water can act as a reducing agent l Water is oxidized to oxygen, O 2 Ø To be stable in aqueous solution, a species must not react with water through a redox process.

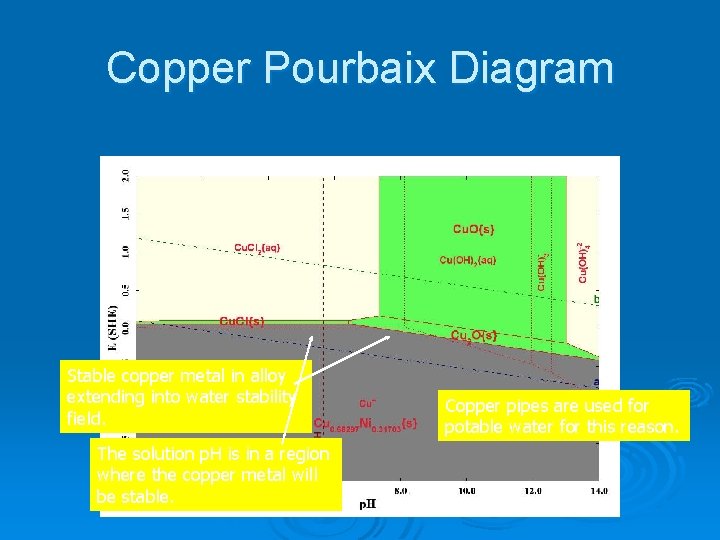
Copper Pourbaix Diagram Stable copper metal in alloy extending into water stability field. The solution p. H is in a region where the copper metal will be stable. Copper pipes are used for potable water for this reason.

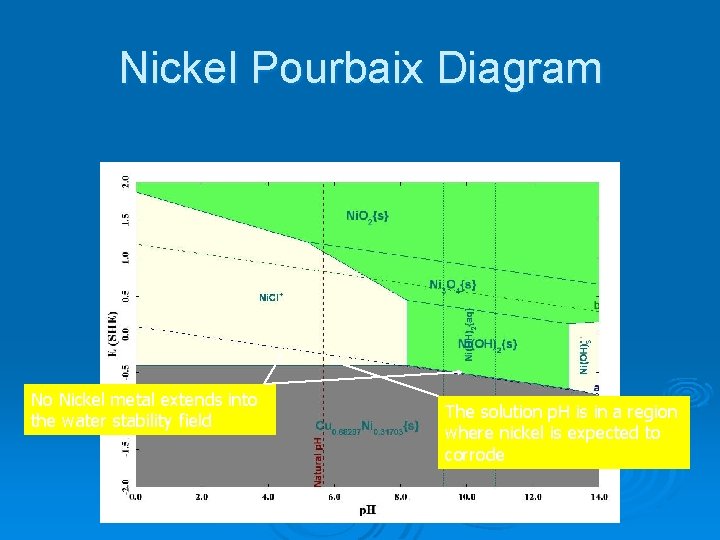
Nickel Pourbaix Diagram No Nickel metal extends into the water stability field The solution p. H is in a region where nickel is expected to corrode

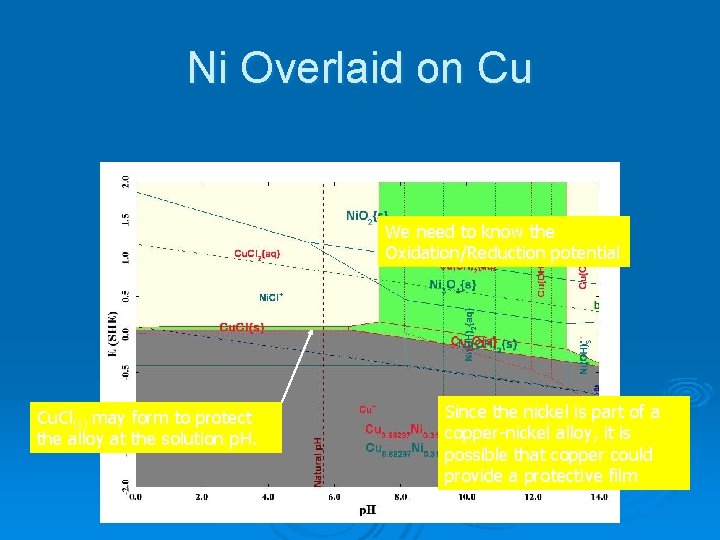
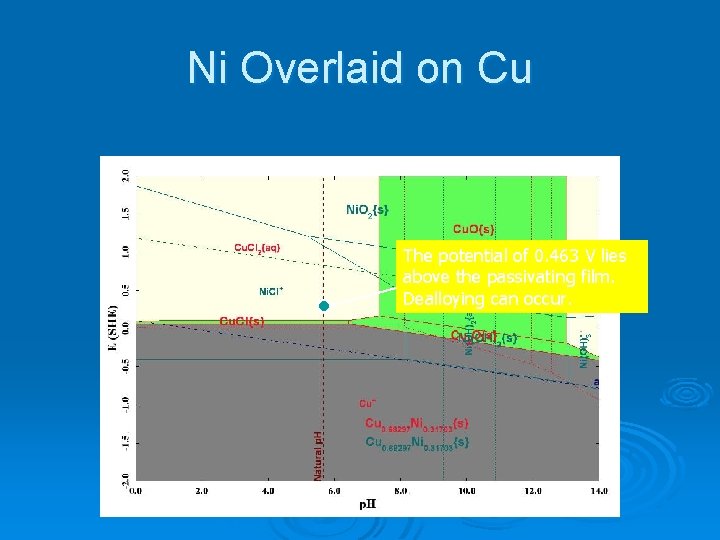
Ni Overlaid on Cu We need to know the Oxidation/Reduction potential Cu. Cl(s) may form to protect the alloy at the solution p. H. Since the nickel is part of a copper-nickel alloy, it is possible that copper could provide a protective film

Application Time

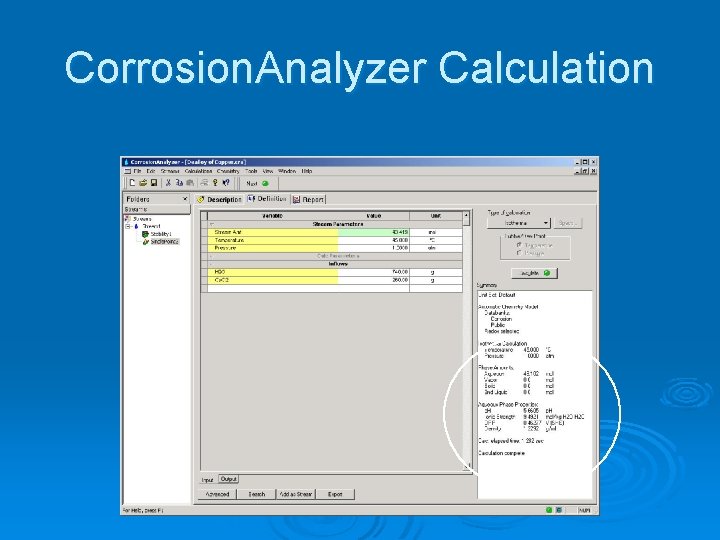
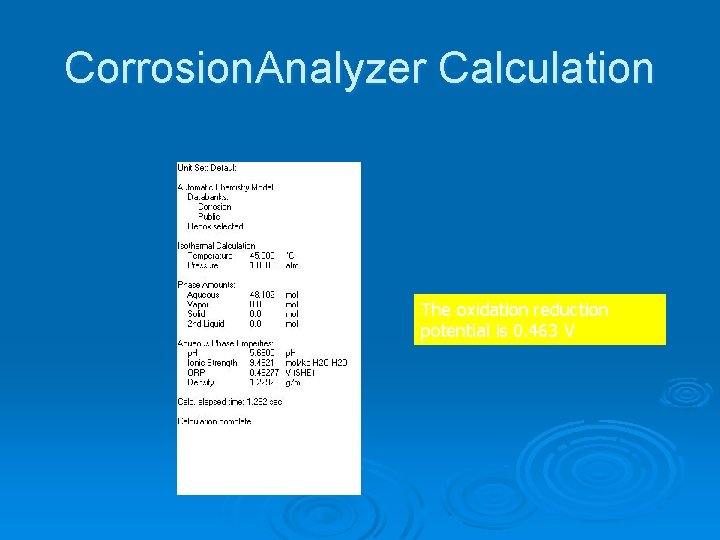
Corrosion. Analyzer Calculation

Corrosion. Analyzer Calculation The oxidation reduction potential is 0. 463 V

Ni Overlaid on Cu The potential of 0. 463 V lies above the passivating film. Dealloying can occur.

Conclusions Ø Why did dealloying occur? l l No protective film at the operating p. H and oxidation/reduction potential of the process fluid. Copper lies within the region of water stability Nickel does not lie within the region of water stability The presence of Cu+ ions in equilibrium with copper metal promotes replating of copper metal driven by the oxidation of nickel.

Chemistry Ø Standard OLI Chemistry l l l 7400 components 9100 individual species 82 Elements of the Periodic Table fully covered • 8 additional elements partially covered. Ø Stability diagrams have access all of this chemistry

Chemistry Ø Alloys l 6 predefined classes supported • • • l Cu Ni Carbon Steels – Fe, Mn, and C Ferritic Stainless steels – Fe, Cr, Ni, Mo and C Austenitic stainless steels Fe, Cr, Ni, Mo and C Duplex stainless steels FCC phase Fe, Cr, Ni, Mo, C and N User defined alloys

Limits to the Standard OLI Chemistry Aqueous Phase XH 2 O > 0. 65 50 o. C < T < 300 o. C 0 Atm < P < 1500 Atm 0 < I < 30 Non aqueous Liquid Currently no Activity Coefficient Model (i. e. , no NRTL, Unifaq/Uniqac) Fugacity Coefficients are determined from the Enhanced SRK

Limitations of Pourbaix Diagrams Ø No information on corrosion kinetics is provided. l Diagram is produced from only thermodynamics. Diagram is valid only for the calculated temperature and pressure Ø Oxide stability fields are calculated thermodynamically and may not provide an actual protective film. Ø Dealloying cannot be predicted from the diagram alone. Ø