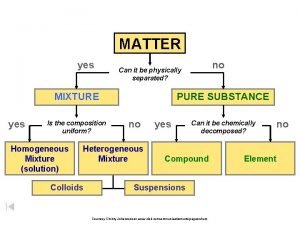
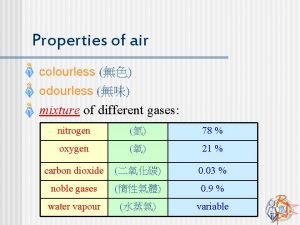
The composition of air Air is a mixture






- Slides: 6

The composition of air

§ Air is a mixture of various substances that is odourless, colourless, or tasteless § Water vapour in the air depends on the humidity in the air. § The more humid the air, the more the water vapour in the air § Examples of inert gases are: - Dust - Water vapour - Microorganisms

§ Air is made up of three main gases: oxygen, carbon dioxide and nitrogen § Each gas has its own chemical properties § The properties of the gases can be observed by : - Solubility in the water - Reaction with sodium hydroxide - Effects on - Glowing wooden splinter Burning wooden splinter Litmus paper Lime water Hydrogen carbonate indicator Nitrogen is a gas that does not react chemically

§ Air that is breathed into the body is called inhaled air § Air that is breathed out of the body is called exhaled air § Oxygen is needed for respiration: § Inhaled oxygen will be dissolved at the surface of the moist alveolus. § Oxygen will be absorbed into the blood capillary through the thin alveolus wall § Oxygen is then transported by red blood cells to the other blood cells for the process of respiration § At the same time, carbon dioxide and water from blood capillaries will be absorbed alveolus. § Carbon dioxide and water will be expelled body when air is exhaled.

§ Combustion is a process that takes place when a substance unites with oxygen chemically and this produces energy and light. § Without oxygen. Combustion cannot occur because chemical process does not take place. § Carbon is a chemical compound that is made up of the carbon element only § Combustion of carbon releases carbon dioxide, heat energy and light energy. § Examples of carbon are wood, cloth, charcoal, and paper. § Carbon + Oxygen Carbon dioxide + Heat energy + Light energy § Hydrocarbon is a chemical compound which is formed from only hydrogen and carbon.

§ Combustion of hydrocarbon produces carbon dioxide, water, heat energy and light energy. § Water is formed when hydrogen from hydrocarbon combines with oxygen during combustion. § Hydrocarbon + Carbon Dioxide +Water +Heat energy + Light energy § Combustion produces light energy and heat energy § Carbon dioxide produced is absorbed by green plants to conduct photosynthesis.