The combined gas law P V T 1
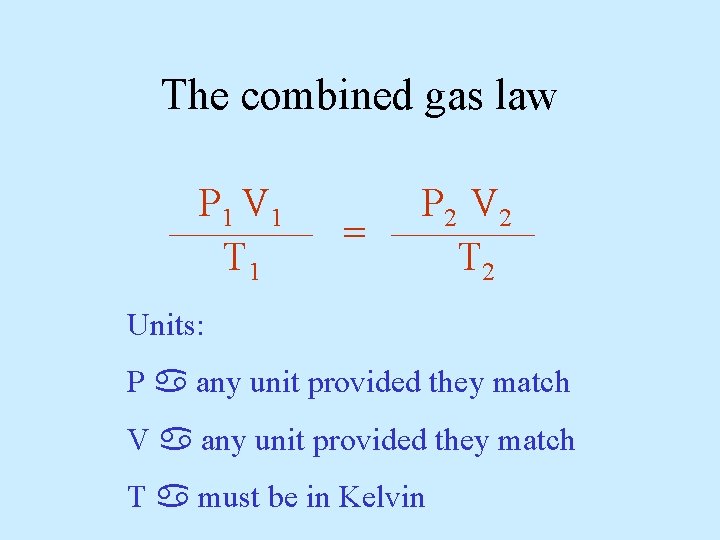
The combined gas law P V T 1 1 1 ______ P V T 2 2 2 ______ = Units: P any unit provided they match V any unit provided they match T must be in Kelvin
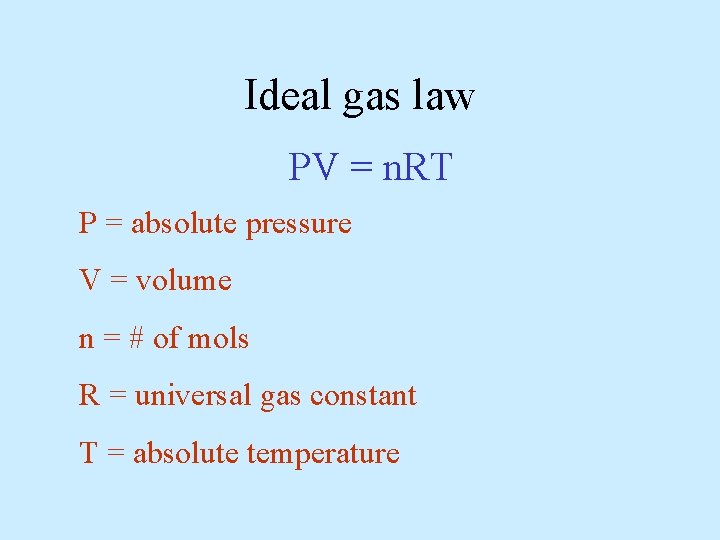
Ideal gas law PV = n. RT P = absolute pressure V = volume n = # of mols R = universal gas constant T = absolute temperature

units: Pressure Volume R Pa m 3 8. 31 J/mol·K T must be in Kelvin n = m/M where: m = mass in grams and M = molar mass in g/mol
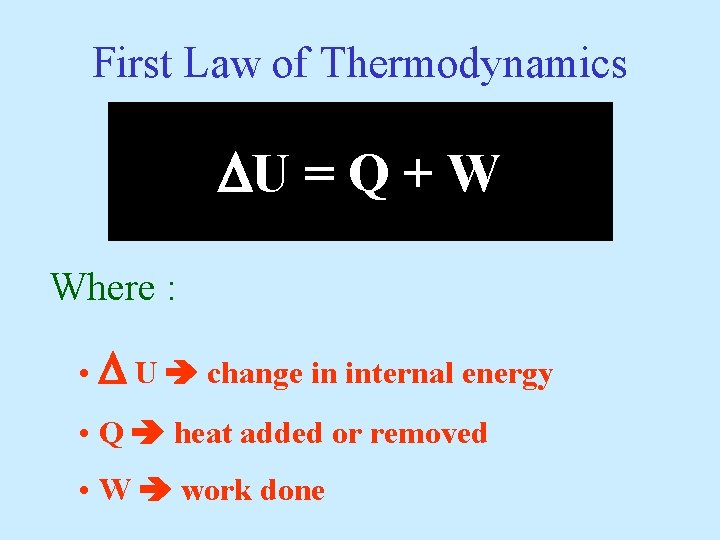
First Law of Thermodynamics U = Q + W Where : • U change in internal energy • Q heat added or removed • W work done
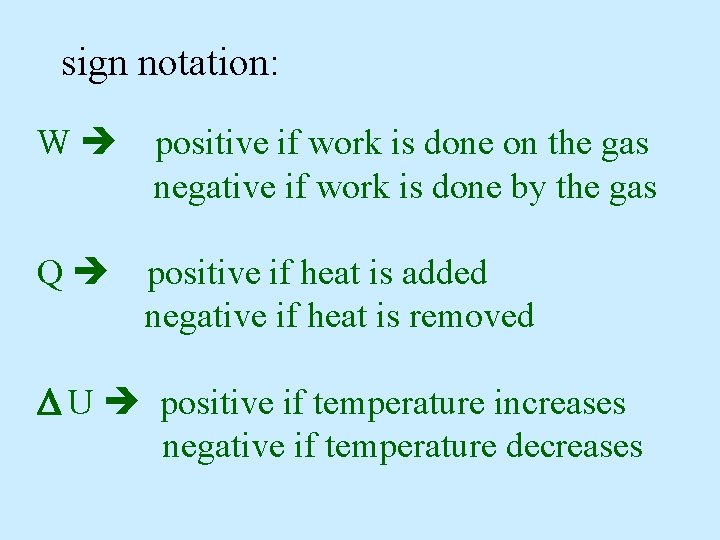
sign notation: W positive if work is done on the gas negative if work is done by the gas Q positive if heat is added negative if heat is removed U positive if temperature increases negative if temperature decreases
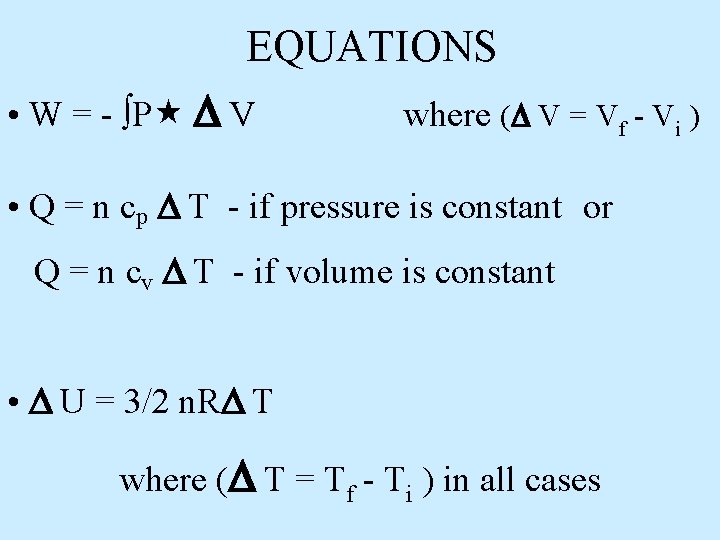
EQUATIONS • W = - ∫P V where ( V = Vf - Vi ) • Q = n cp T - if pressure is constant or Q = n cv T - if volume is constant • U = 3/2 n. R T where ( T = Tf - Ti ) in all cases

CONSTANTS • cp = 5/2 R ( 20. 775 J/K ) • cv = 3/2 R ( 12. 465 J/K ) • R = 8. 31 J/mol·K

UNITS W JOULES Q JOULES U JOULES Therefore: Pressure must be in Pa Volume must be in m 3 R is 8. 31 J/mol K
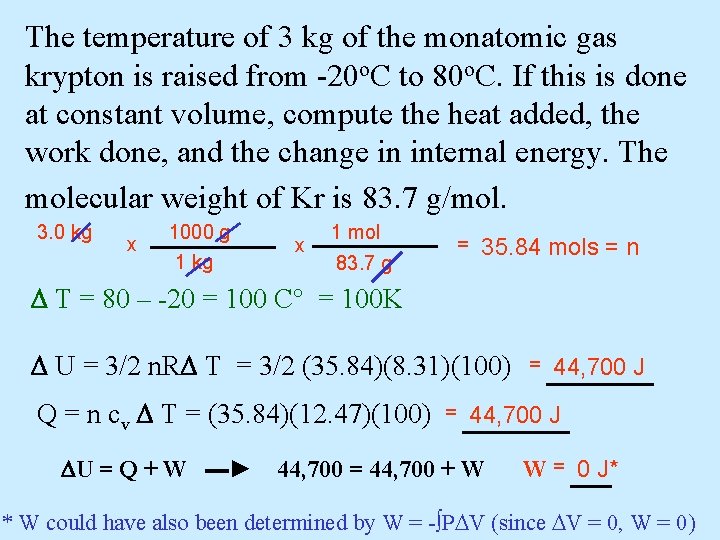
The temperature of 3 kg of the monatomic gas krypton is raised from -20 o. C to 80 o. C. If this is done at constant volume, compute the heat added, the work done, and the change in internal energy. The molecular weight of Kr is 83. 7 g/mol. 3. 0 kg x 1000 g 1 kg x 1 mol 83. 7 g = 35. 84 mols = n T = 80 – -20 = 100 C° = 100 K U = 3/2 n. R T = 3/2 (35. 84)(8. 31)(100) = 44, 700 J Q = n cv T = (35. 84)(12. 47)(100) = 44, 700 J U = Q + W ▬► 44, 700 = 44, 700 + W W = 0 J* * W could have also been determined by W = -∫P V (since V = 0, W = 0)
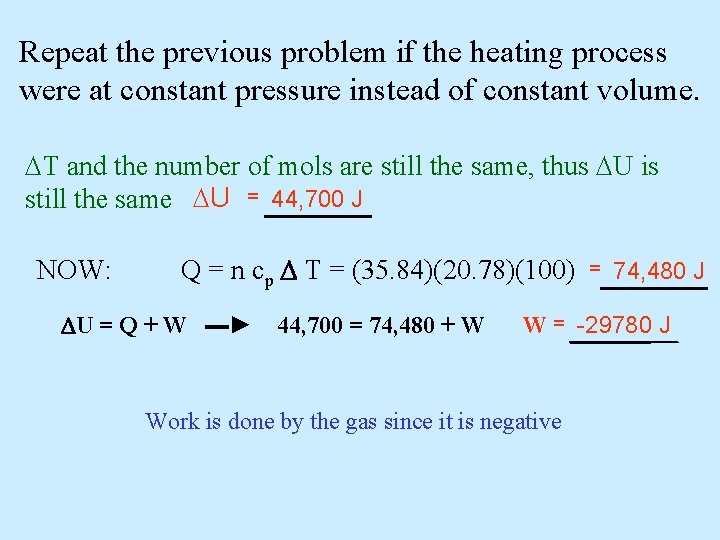
Repeat the previous problem if the heating process were at constant pressure instead of constant volume. T and the number of mols are still the same, thus U is still the same U = 44, 700 J NOW: Q = n cp T = (35. 84)(20. 78)(100) = 74, 480 J U = Q + W ▬► 44, 700 = 74, 480 + W W = -29780 J Work is done by the gas since it is negative
- Slides: 10