The CLASSICS trial study design 8 European countries
- Slides: 5

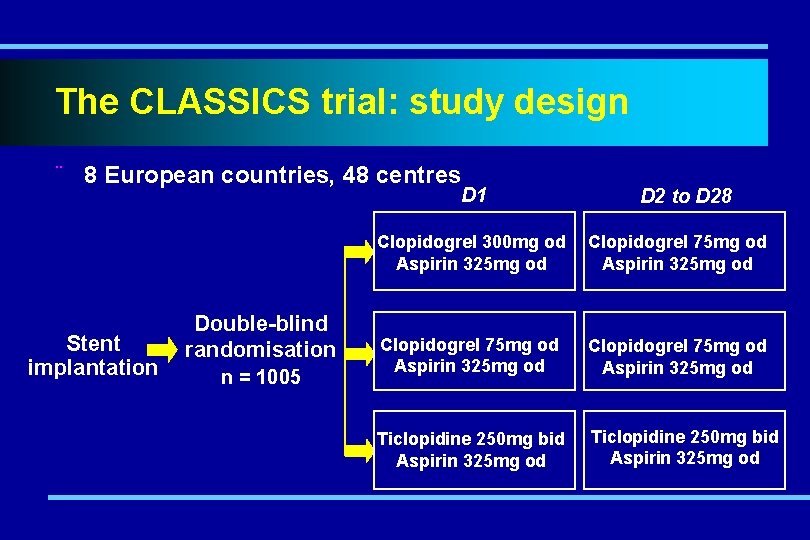
The CLASSICS trial: study design ¨ 8 European countries, 48 centres Stent implantation Double-blind randomisation n = 1005 D 1 D 2 to D 28 Clopidogrel 300 mg od Aspirin 325 mg od Clopidogrel 75 mg od Aspirin 325 mg od Ticlopidine 250 mg bid Aspirin 325 mg od

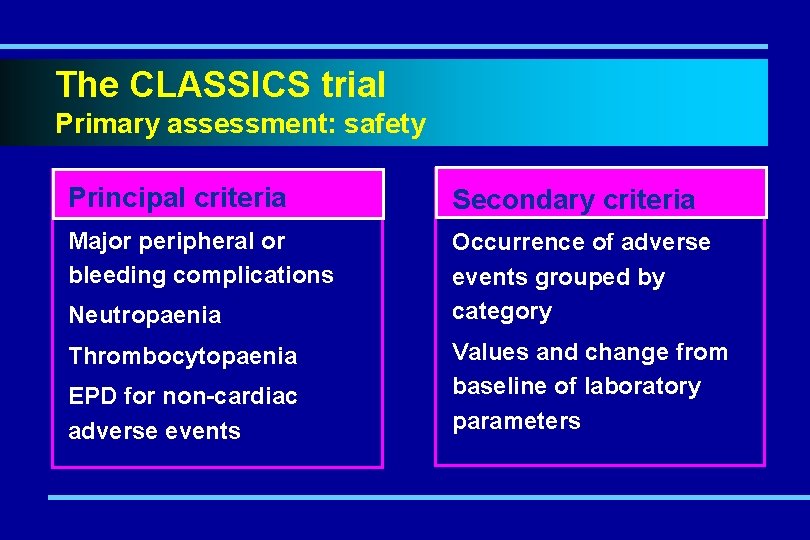
The CLASSICS trial Primary assessment: safety Principal criteria Secondary criteria Major peripheral or bleeding complications Occurrence of adverse events grouped by category Neutropaenia Thrombocytopaenia EPD for non-cardiac adverse events Values and change from baseline of laboratory parameters

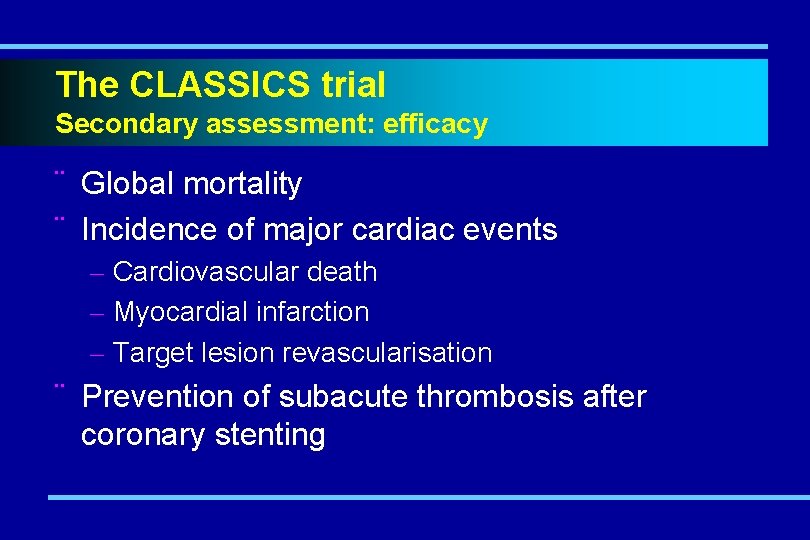
The CLASSICS trial Secondary assessment: efficacy ¨ Global mortality ¨ Incidence of major cardiac events – Cardiovascular death – Myocardial infarction – Target lesion revascularisation ¨ Prevention of subacute thrombosis after coronary stenting

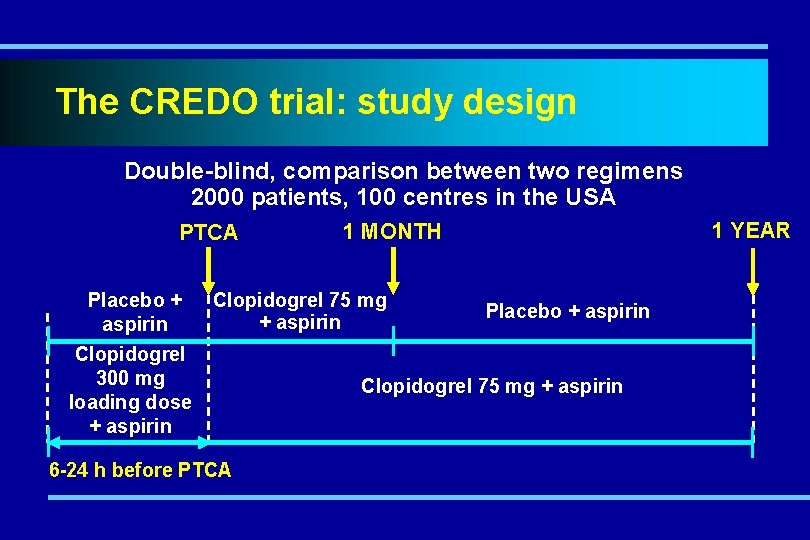
The CREDO trial: study design Double-blind, comparison between two regimens 2000 patients, 100 centres in the USA PTCA Placebo + aspirin Clopidogrel 75 mg + aspirin Clopidogrel 300 mg loading dose + aspirin 6 -24 h before PTCA 1 YEAR 1 MONTH Placebo + aspirin Clopidogrel 75 mg + aspirin

Clopidogrel for coronary stenting: current status ¨ Use of stenting in coronary angioplasty is increasing ¨ Complementary antithrombotic and antiplatelet therapies are becoming established to reduce the risk of subacute thrombosis after coronary stenting ¨ Ticlopidine + aspirin have proven antiplatelet efficacy in coronary stenting ¨ The CLASSICS trial compares the safety of ticlopidine + aspirin with clopidogrel (with and without a loading dose) + aspirin for coronary stenting ¨ The CREDO trial is underway to assess the efficacy of clopidogrel + aspirin at 30 days and 1 year following PTCA, with or without stenting