The chemists tools Elements and the periodic table

The chemists tools Elements and the periodic table
Elements l Pure substances that cannot be separated into different substances by ordinary processes l Are the building blocks of matter l 120 elements known today Examples: carbon gold calcium
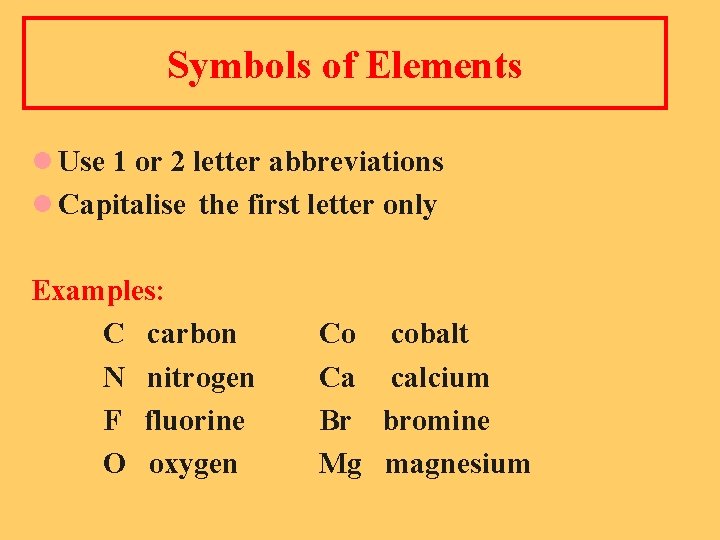
Symbols of Elements l Use 1 or 2 letter abbreviations l Capitalise the first letter only Examples: C carbon N nitrogen F fluorine O oxygen Co Ca Br Mg cobalt calcium bromine magnesium

Symbols from Latin Names Element Copper Gold Lead Mercury Potassium Silver Sodium Tin Symbol Cu Au Pb Hg K Ag Na Sn Latin name cuprum aurum plumbum hydrargyrum kalium argentum natrium stannum
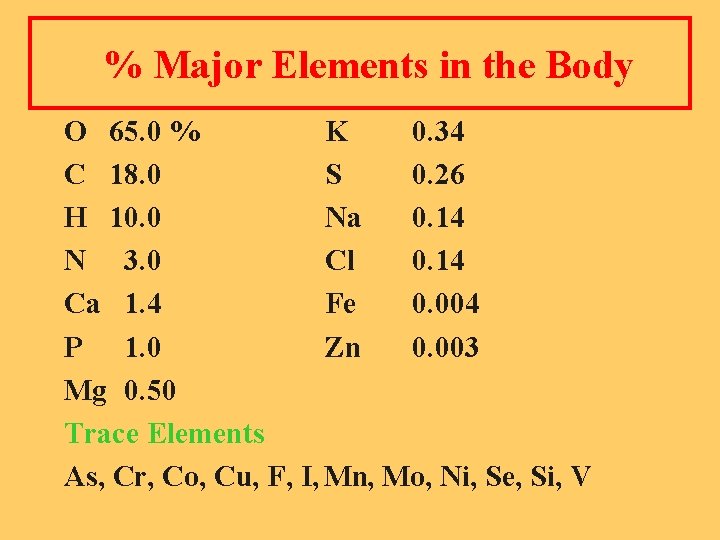
% Major Elements in the Body O 65. 0 % K 0. 34 C 18. 0 S 0. 26 H 10. 0 Na 0. 14 N 3. 0 Cl 0. 14 Ca 1. 4 Fe 0. 004 P 1. 0 Zn 0. 003 Mg 0. 50 Trace Elements As, Cr, Co, Cu, F, I, Mn, Mo, Ni, Se, Si, V
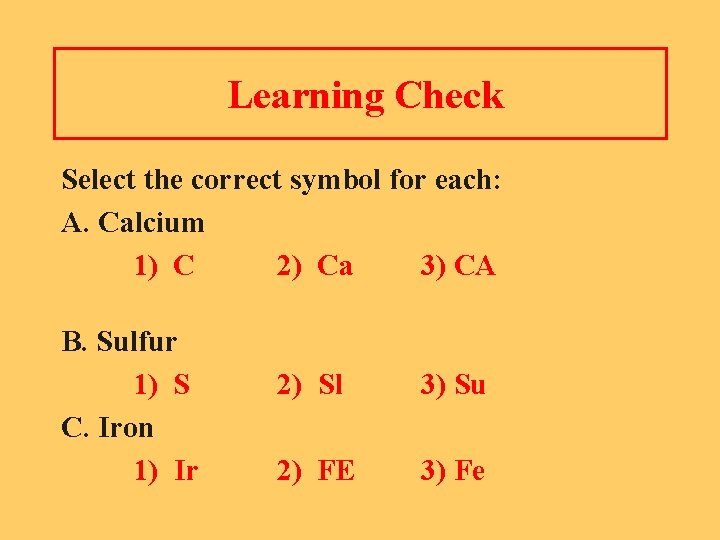
Learning Check Select the correct symbol for each: A. Calcium 1) C 2) Ca 3) CA B. Sulfur 1) S C. Iron 1) Ir 2) Sl 3) Su 2) FE 3) Fe

Solution Select the correct symbol for each: A. Calcium 2) Ca B. Sulfur 1) S C. Iron 3) Fe

Learning Check Select the correct name for each: A. N 1) neon 2) nitrogen 3) nickel B. P 1) potassium 2) phlogiston 3) phosphorus C. Ag 1) silver 2) aegean 3) gold

Solution Select the correct name for each: A. N 2) nitrogen B. P 3) phosphorus C. Ag 1) silver
Periodic Table The periodic table is an arrangement of the elements according to similarities in their chemical and physical properties.

Physical Properties The characteristics of a substance that can be observed without changing the substance. n Color n Size n Shape n Density n Freezing and Boiling Points n Odor
Groups of Elements l Vertical columns on the periodic table l Similar physical properties l Similar chemical properties
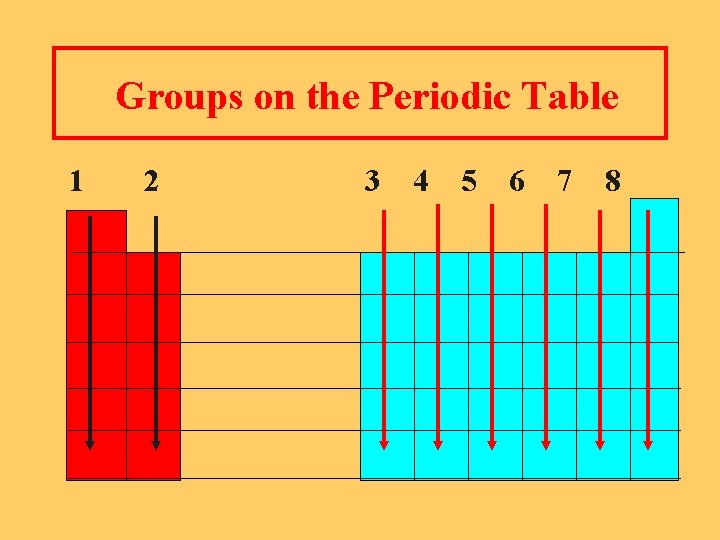
Groups on the Periodic Table 1 2 3 4 5 6 7 8
Representative Groups l Group 1 Alkali Metals l Group 2 Alkaline Earth Metals l Group 7 Halogens l Group 8 Noble Gases
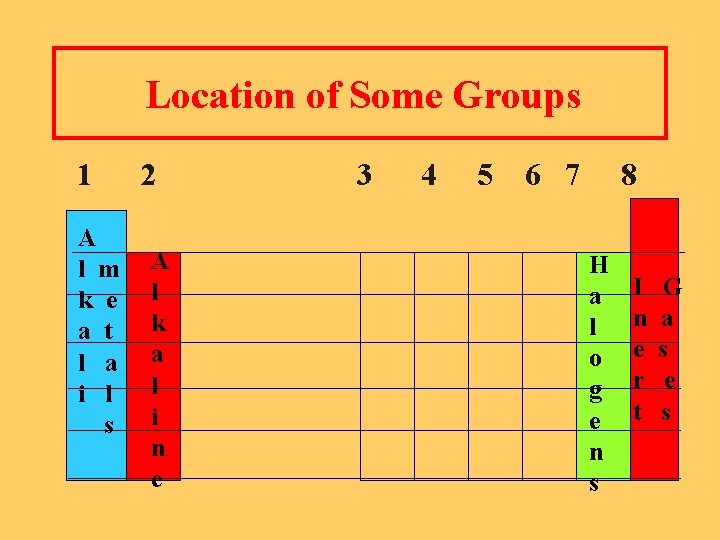
Location of Some Groups 1 A l m k e a t l a i l s 2 A l k a l i n e 3 4 5 6 7 8 H a l o g e n s I n e r t G a s e s
Periods on the Periodic Table l Horizontal rows from Group 1 to Group 8. l Numbered 1, 2, 3, …. l Include representative elements and transition elements
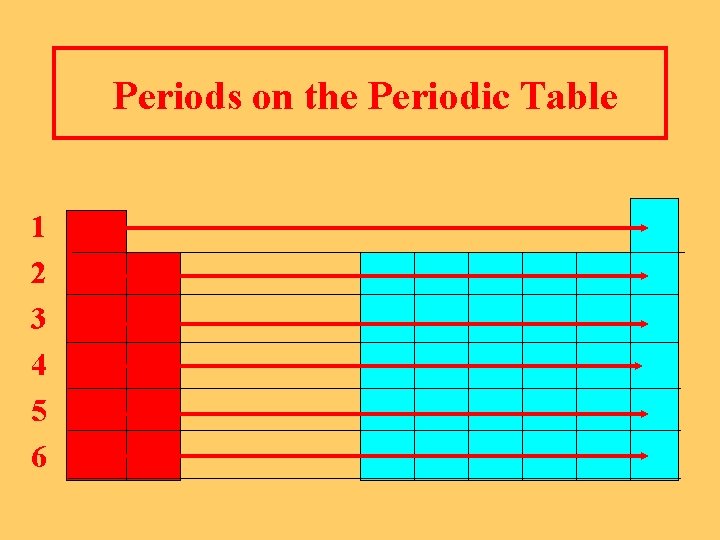
Periods on the Periodic Table 1 2 3 4 5 6
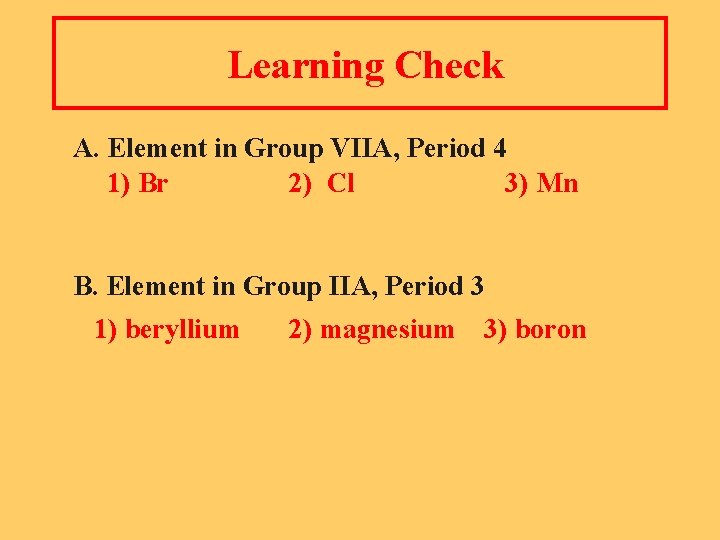
Learning Check A. Element in Group VIIA, Period 4 1) Br 2) Cl 3) Mn B. Element in Group IIA, Period 3 1) beryllium 2) magnesium 3) boron

Solution A. Element in Group 7 A, period 4 1) Br B. Element in Group 2 A, Period 3 2) magnesium
Learning Check A. Element in Group VIIA, period 4 1) Br 2) Cl 3) Mn B. Element in Group IIA, Period 3 1) beryllium 2) magnesium C. Metals in Group IVA 1) Ge, Sn, Pb 2) C, Si 3) boron 3) C, Si, Ge, Sn D. Nonmetals in Group VA 1) As, Sb, Bi 2) N, P, As 3) N, P, As, Sb

Solution A. Element in Group VIIA, period 4 1) Br B. Element in Group IIA, Period 3 2) magnesium C. Metals in Group IVA 1) Ge, Sn, Pb D. Nonmetals in Group VA 2) N, P, As

Metals and Nonmetals Metals n Located to the left of the heavy line n Shiny, ductile n Good conductors of heat and electricity Nonmetals n Located to the right of the heavy line n Dull and brittle n Poor conductors, good insulators
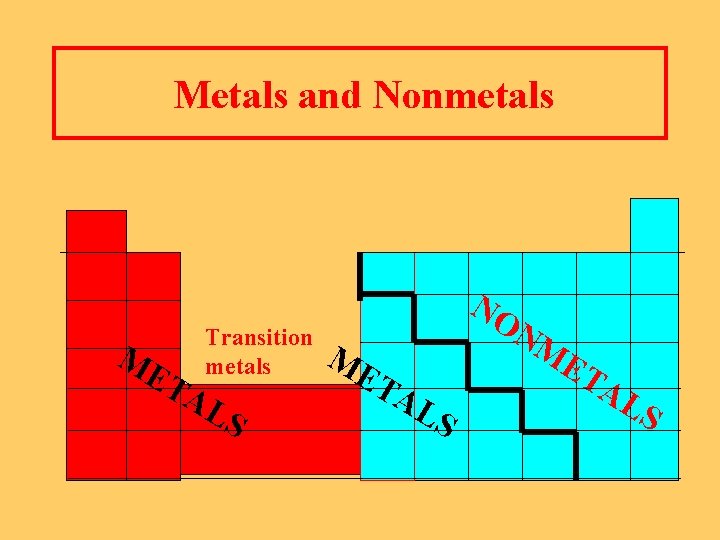
Metals and Nonmetals ME TA Transition metals LS NO ME TA LS NM ET AL S
Learning Check Specify metal (1) A. sulphur B. chlorine C. sodium D. iron E. carbon F. silver or nonmetal (2) for each: ____ ____

Solution Specify metal (1) A. sulphur B. chlorine C. sodium D. iron E. carbon F. silver or nonmetal (2) for each: 2 2 1 1 2 1

Learning Check Select the correct elements: A. Metals in Group IVA 1) Ge, Sn, Pb 2) C, Si 3) C, Si, Ge, Sn B. Non-metals in Group VA 1) As, Sb, Bi 2) N, P, As 3) N, P, As, Sb

Solution A. Metals in Group 4 A 1) Ge, Sn, Pb B. Nonmetals in Group 5 A 2) N, P, As
- Slides: 27