The chemistry of vanadium Variable Oxidation States What
The chemistry of vanadium

Variable Oxidation States • What type of reaction is it when you switch between oxidation states? • Redox reaction • The metal ions are either oxidised or reduced
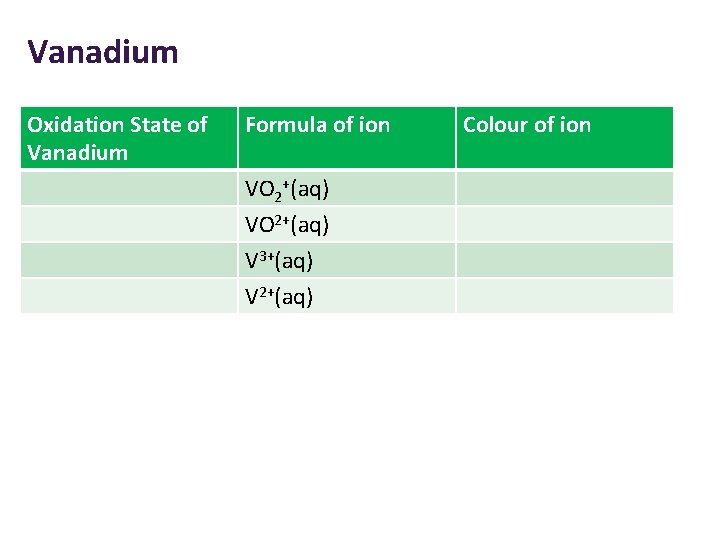
Vanadium Oxidation State of Vanadium Formula of ion VO 2+(aq) V 3+(aq) V 2+(aq) Colour of ion
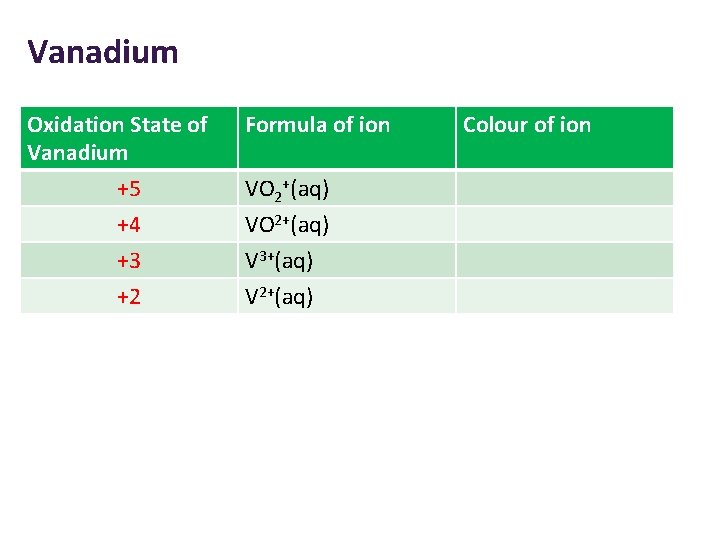
Vanadium Oxidation State of Vanadium +5 +4 +3 +2 Formula of ion VO 2+(aq) V 3+(aq) V 2+(aq) Colour of ion
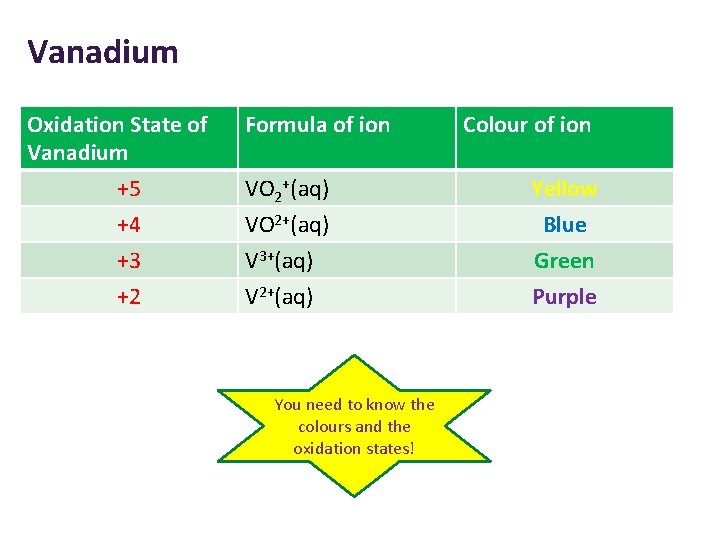
Vanadium Oxidation State of Vanadium +5 +4 +3 +2 Formula of ion Colour of ion VO 2+(aq) Yellow VO 2+(aq) V 3+(aq) V 2+(aq) Blue Green Purple You need to know the colours and the oxidation states!
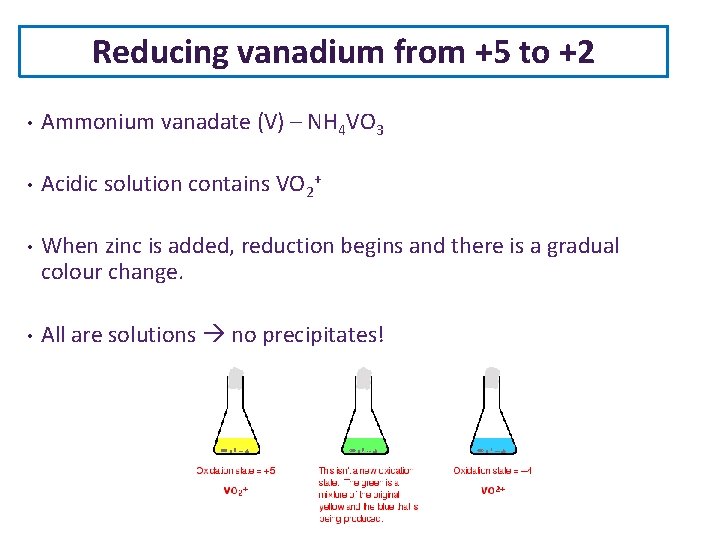
Reducing vanadium from +5 to +2 • Ammonium vanadate (V) – NH 4 VO 3 • Acidic solution contains VO 2+ • When zinc is added, reduction begins and there is a gradual colour change. • All are solutions no precipitates!
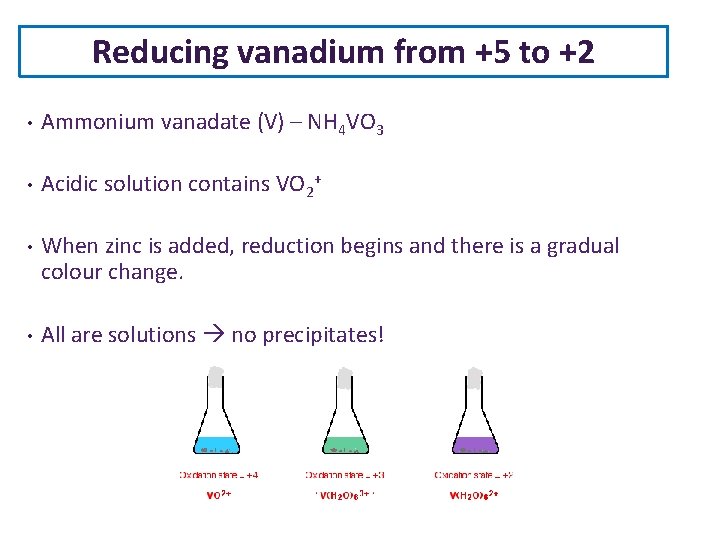
Reducing vanadium from +5 to +2 • Ammonium vanadate (V) – NH 4 VO 3 • Acidic solution contains VO 2+ • When zinc is added, reduction begins and there is a gradual colour change. • All are solutions no precipitates!
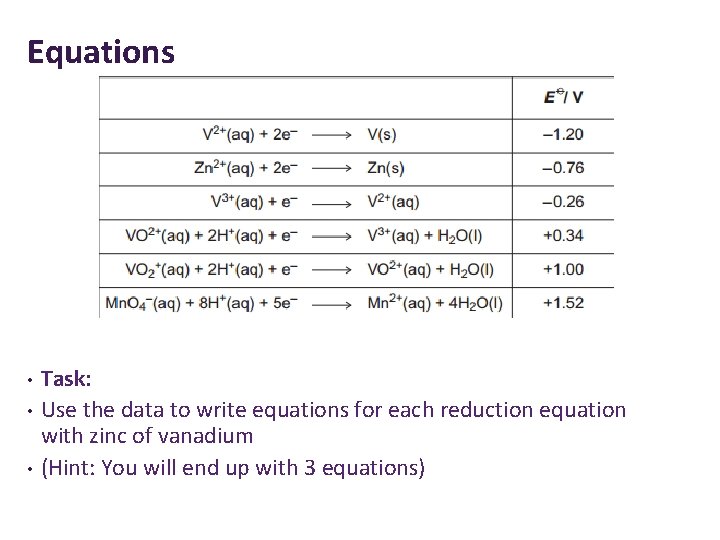
Equations • • • Task: Use the data to write equations for each reduction equation with zinc of vanadium (Hint: You will end up with 3 equations)

Equations 1. Yellow to blue Vanadium(V) to vanadium(IV) 2 VO 2+(aq) +Zn(s) +4 H+(aq) → 2 VO 2+(aq) +Zn 2+(aq) +2 H 2 O(l) 2. Blue to green Vanadium(IV) to vanadium(III) 2 VO 2+(aq) +Zn(s) +4 H+(aq) → 2 V 3+(aq) +Zn 2+(aq) +2 H 2 O(l) 3. Green to Purple Vanadium(III) to vanadium(II) 2 V 3+(aq) +Zn(s) → 2 V 2+(aq) +Zn 2+(aq)

EXAM QUESTION
- Slides: 10