The Chemistry of Swimming History of Swimming As










- Slides: 18

The Chemistry of Swimming.

History of Swimming § As early as the Stone Age, people swam. § Ancient Greeks were the first people who practiced swimming as a § § sport. Swimming was one of only 5 sports competed in at the first Modern Olympic Games in 1896. Other Olympic pool events include water polo, synchronised swimming and diving.

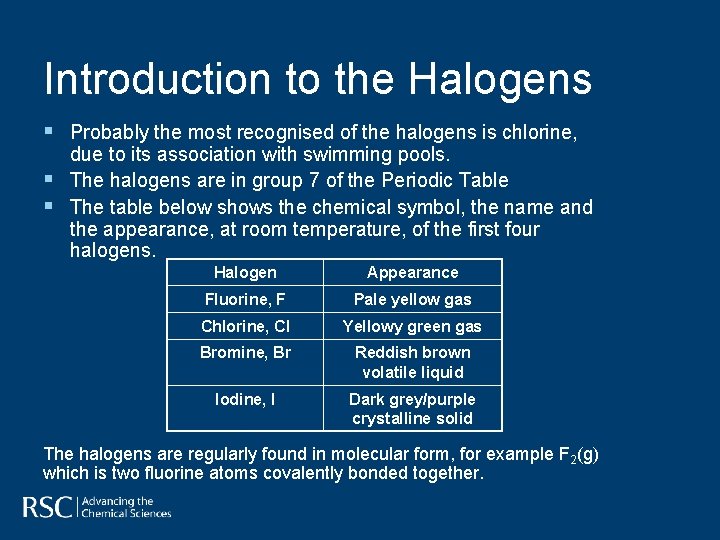
Introduction to the Halogens § Probably the most recognised of the halogens is chlorine, § § due to its association with swimming pools. The halogens are in group 7 of the Periodic Table The table below shows the chemical symbol, the name and the appearance, at room temperature, of the first four halogens. Halogen Appearance Fluorine, F Pale yellow gas Chlorine, Cl Yellowy green gas Bromine, Br Reddish brown volatile liquid Iodine, I Dark grey/purple crystalline solid The halogens are regularly found in molecular form, for example F 2(g) which is two fluorine atoms covalently bonded together.

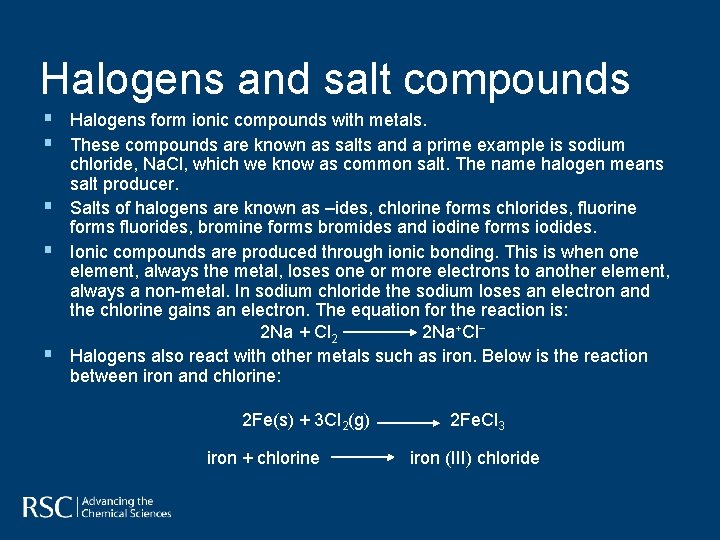
Halogens and salt compounds § Halogens form ionic compounds with metals. § These compounds are known as salts and a prime example is sodium § § § chloride, Na. Cl, which we know as common salt. The name halogen means salt producer. Salts of halogens are known as –ides, chlorine forms chlorides, fluorine forms fluorides, bromine forms bromides and iodine forms iodides. Ionic compounds are produced through ionic bonding. This is when one element, always the metal, loses one or more electrons to another element, always a non-metal. In sodium chloride the sodium loses an electron and the chlorine gains an electron. The equation for the reaction is: 2 Na + Cl 2 2 Na+Cl− Halogens also react with other metals such as iron. Below is the reaction between iron and chlorine: 2 Fe(s) + 3 Cl 2(g) iron + chlorine 2 Fe. Cl 3 iron (III) chloride

Answers to worksheet 1. Chlorine, the chemical so readily associated with the swimming pool, can also be found in common salt. What is the chemical name and chemical formula for salt? Sodium chloride, Na. Cl 2. Fluorine, chlorine, and bromine are all group 7 elements. What is another name given to this group of elements? The ‘halogens’ 3. In the table below write the chemical symbol corresponding to the element and also the appearance of that element when in molecular form at room temperature. Fluorine has been included as the example. (Answers are in red!!) Element Symbol Appearance Fluorine F Pale yellow gas Chlorine Bromine Iodine

Answers to worksheet 1. Chlorine, the chemical so readily associated with the swimming pool, can also be found in common salt. What is the chemical name and chemical formula for salt? Sodium chloride, Na. Cl 2. Fluorine, chlorine, and bromine are all group 7 elements. What is another name given to this group of elements? The ‘halogens’ 3. In the table below write the chemical symbol corresponding to the element and also the appearance of that element when in molecular form at room temperature. Fluorine has been included as the example. (Answers are in red!!) Element Symbol Appearance Fluorine F Pale yellow gas Chlorine Cl Yellowy green gas Bromine Br Reddish brown volatile liquid Iodine I Dark grey/purple crystalline solid

Answers to worksheet 4. What type of bonding has occurred when two chlorine atoms have bonded together to form a molecule of chlorine? Covalent bonding 5. In question 1 you wrote down the chemical formula for salt. Identify which atom has lost an electron and which atom has gained an electron to form the ionic bond in the compound. The sodium atom has lost the electron and the chlorine atom has gained the electron. 6. A common chloride encountered is iron (III) chloride. Balance the following equation between iron and chlorine to produce iron (III) chloride. 2 Fe(s) + 3 Cl 2(g) 2 Fe. Cl 3(s) 4. At room temperature, only one of the first four halogens can be found in a solid state. This halogen, when in a solution, can be used to test for starch. What is the halogen? What colour does the starch go when tested with this halogen? The solution will turn blue/black if starch is present and the halogen is iodine.

Energy Levels § Each element at atomic level is made up of protons, neutrons and electrons - except for hydrogen, which is a special case and has no neutrons. § The protons and neutrons are clustered together at the centre of the atom known as the nucleus. The electrons move around the outside of the nucleus at certain distances from the nucleus. These levels are known as energy levels and you may sometimes hear them referred to as ‘shells’. § Each energy level can hold a certain number of electrons before becoming ‘full’. Once full the next energy level away from the nucleus is filled, and so on. § Atoms are considered to be stable when their energy levels are full and in some cases are relatively stable when energy levels are filled to a certain amount. Atoms will look to gain or lose electrons in order to reach these states of stability in their energy levels. This is why ionic bonding occurs!

Energy Levels § Different energy levels can hold a different number of electrons before being considered to be full: § The first energy level can hold 2 electrons (once this energy level is full electrons start to fill the second energy level). § The second energy level can hold 8 electrons (the second energy level is filled after the first energy level but before third energy level). § The third energy level can hold 18 electrons (the energy level is also relatively stable when containing 8 electrons).

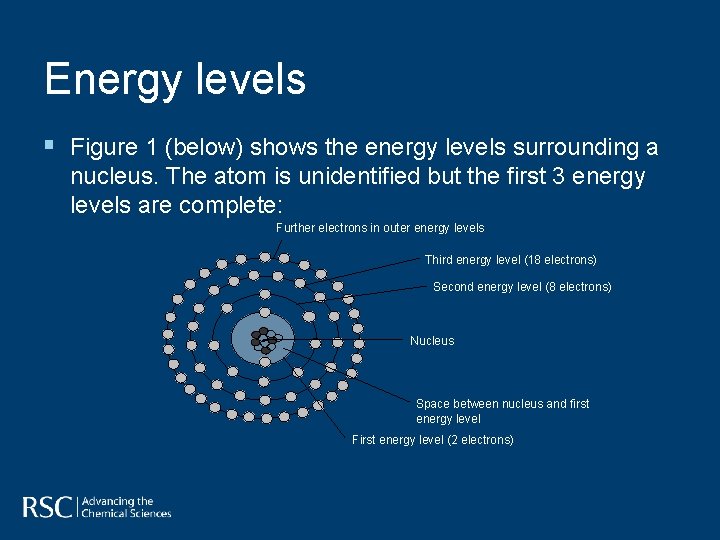
Energy levels § Figure 1 (below) shows the energy levels surrounding a nucleus. The atom is unidentified but the first 3 energy levels are complete: Further electrons in outer energy levels Third energy level (18 electrons) Second energy level (8 electrons) Nucleus Space between nucleus and first energy level First energy level (2 electrons)

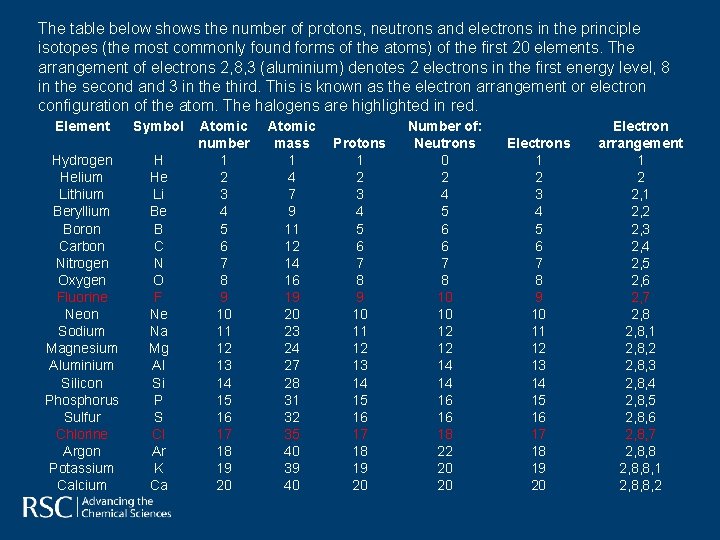
The table below shows the number of protons, neutrons and electrons in the principle isotopes (the most commonly found forms of the atoms) of the first 20 elements. The arrangement of electrons 2, 8, 3 (aluminium) denotes 2 electrons in the first energy level, 8 in the second and 3 in the third. This is known as the electron arrangement or electron configuration of the atom. The halogens are highlighted in red. Element Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium Symbol H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Atomic number 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Atomic mass 1 4 7 9 11 12 14 16 19 20 23 24 27 28 31 32 35 40 39 40 Protons 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Number of: Neutrons 0 2 4 5 6 6 7 8 10 10 12 12 14 14 16 16 18 22 20 20 Electrons 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Electron arrangement 1 2 2, 1 2, 2 2, 3 2, 4 2, 5 2, 6 2, 7 2, 8, 1 2, 8, 2 2, 8, 3 2, 8, 4 2, 8, 5 2, 8, 6 2, 8, 7 2, 8, 8, 1 2, 8, 8, 2

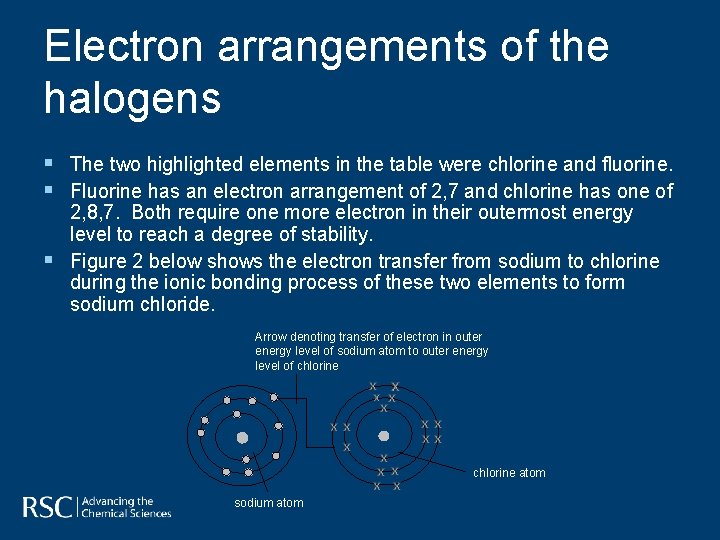
Electron arrangements of the halogens § The two highlighted elements in the table were chlorine and fluorine. § Fluorine has an electron arrangement of 2, 7 and chlorine has one of § 2, 8, 7. Both require one more electron in their outermost energy level to reach a degree of stability. Figure 2 below shows the electron transfer from sodium to chlorine during the ionic bonding process of these two elements to form sodium chloride. Arrow denoting transfer of electron in outer energy level of sodium atom to outer energy level of chlorine atom sodium atom

Chlorine in swimming pools § Chlorine is added to swimming pools to kill harmful germs and bacteria. § It is rarely added in the form of a gas but more commonly in § § compound or solution form. HOCl is called chloric(I) acid and is the main agent added to pool water. It is normally added in the form of sodium or calcium hypochlorite which easily dissociates to form HOCl or ‘free chlorine’. It is this free chlorine that breaks down bacteria by entering the cell walls and disrupting protein and enzyme functions. Chloric(I) acid also reacts with ammonia, something present in sweat and urine, to form chloroamines. It is in fact dichloroamine that gives swimming pools that distinctive ‘chlorine’ smell. To break down chloroamines more chlorine should be added to cause a shock reaction. Oddly the ‘chlorine’ smell is due to too little chlorine being present rather than too much.

Answers to revision sheet 1. 2. 3. In which group of the Periodic Table are the halogens? Group 7 What are the first four halogens in the Periodic Table? Fluorine, chlorine, bromine and iodine. Fluorine is found at room temperature as a gas, F 2. How are the two fluorine atoms bonded? Highlight a or b. a) covalent bond 4. or b) ionic bond Fill in the chemical symbol or element name, the atomic number and number of protons, neutrons and electrons, where appropriate, in the table below for the first 20 elements of the Periodic Table. Leave the gaps in the final column (called electron arrangement) empty at the moment. Please see the table on the next slide for the answers.

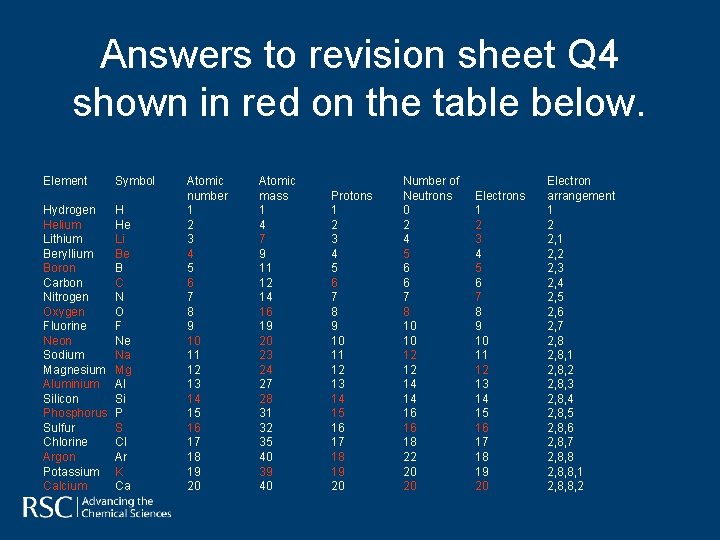
Answers to revision sheet Q 4 shown in red on the table below. Element Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium Symbol H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Atomic number 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Atomic mass 1 4 7 9 11 12 14 16 19 20 23 24 27 28 31 32 35 40 39 40 Protons 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Number of Neutrons 0 2 4 5 6 6 7 8 10 10 12 12 14 14 16 16 18 22 20 20 Electrons 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Electron arrangement 1 2 2, 1 2, 2 2, 3 2, 4 2, 5 2, 6 2, 7 2, 8, 1 2, 8, 2 2, 8, 3 2, 8, 4 2, 8, 5 2, 8, 6 2, 8, 7 2, 8, 8, 1 2, 8, 8, 2

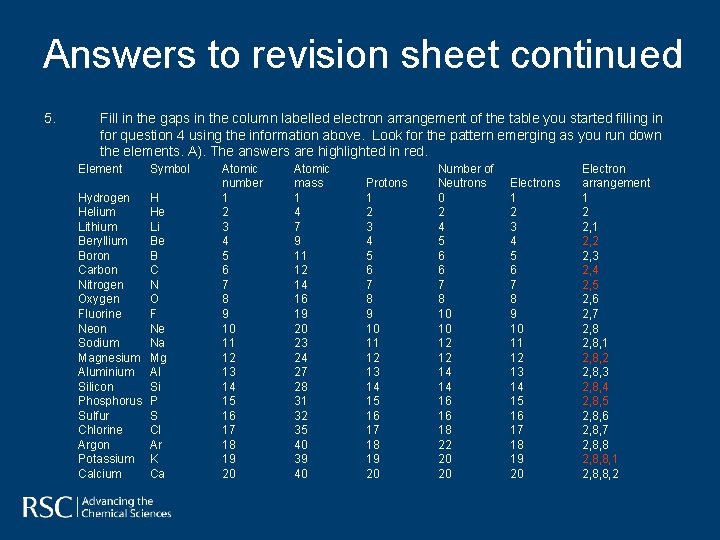
Answers to revision sheet continued 5. Fill in the gaps in the column labelled electron arrangement of the table you started filling in for question 4 using the information above. Look for the pattern emerging as you run down the elements. A). The answers are highlighted in red. Element Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium Symbol H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Atomic number 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Atomic mass 1 4 7 9 11 12 14 16 19 20 23 24 27 28 31 32 35 40 39 40 Protons 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Number of Neutrons 0 2 4 5 6 6 7 8 10 10 12 12 14 14 16 16 18 22 20 20 Electrons 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Electron arrangement 1 2 2, 1 2, 2 2, 3 2, 4 2, 5 2, 6 2, 7 2, 8, 1 2, 8, 2 2, 8, 3 2, 8, 4 2, 8, 5 2, 8, 6 2, 8, 7 2, 8, 8, 1 2, 8, 8, 2

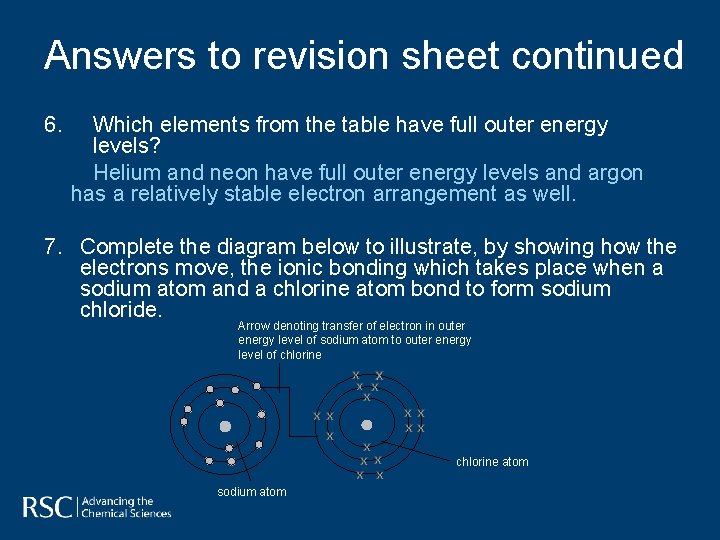
Answers to revision sheet continued 6. Which elements from the table have full outer energy levels? Helium and neon have full outer energy levels and argon has a relatively stable electron arrangement as well. 7. Complete the diagram below to illustrate, by showing how the electrons move, the ionic bonding which takes place when a sodium atom and a chlorine atom bond to form sodium chloride. Arrow denoting transfer of electron in outer energy level of sodium atom to outer energy level of chlorine atom sodium atom

Answers to revision sheet continued 8. 9. What is the maximum number of electrons which can be held in the third energy level? 18 electrons can be held in the third energy level. Why is chlorine used in swimming pools? Highlight a, b or c. a) To colour the water b) To make your eyes sting c) To kill harmful germs and bacteria 10. Dichloroamines are actually what give swimming pools that ‘chlorine’ smell. To remove dichloroamines, shock treatment is required which breaks down these dichloroamines leaving a slight excess of chlorine. Does shock treatment require more or less chlorine to be added to swimming pools to remove the ‘chlorine’ smell? More chlorine needs to be added to remove the so called ‘chlorine’ smell.
 Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật