The Chemistry of Microbiology CHAPTER 2 Atoms Matter

The Chemistry of Microbiology CHAPTER 2
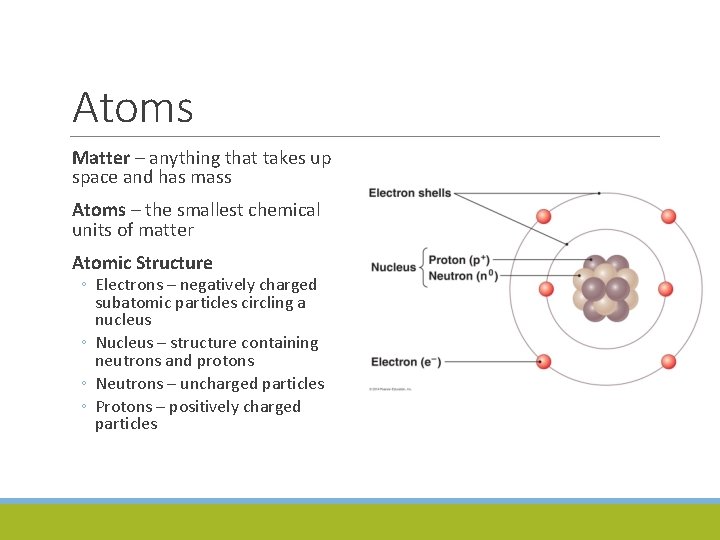
Atoms Matter – anything that takes up space and has mass Atoms – the smallest chemical units of matter Atomic Structure ◦ Electrons – negatively charged subatomic particles circling a nucleus ◦ Nucleus – structure containing neutrons and protons ◦ Neutrons – uncharged particles ◦ Protons – positively charged particles
Atoms Atomic Structure ◦ Element – composed of a single type of atom ◦ Atomic number – equal to the number of protons in the nucleus ◦ Atomic mass (atomic weight) – sum of masses of protons, neutrons, and electrons
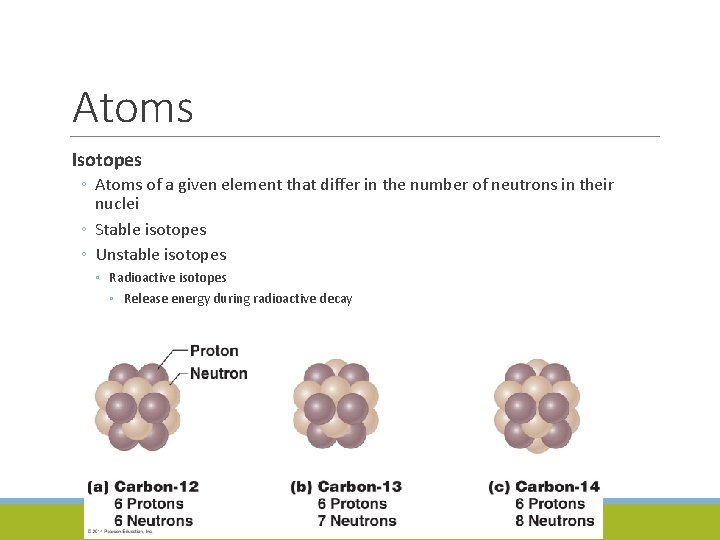
Atoms Isotopes ◦ Atoms of a given element that differ in the number of neutrons in their nuclei ◦ Stable isotopes ◦ Unstable isotopes ◦ Radioactive isotopes ◦ Release energy during radioactive decay
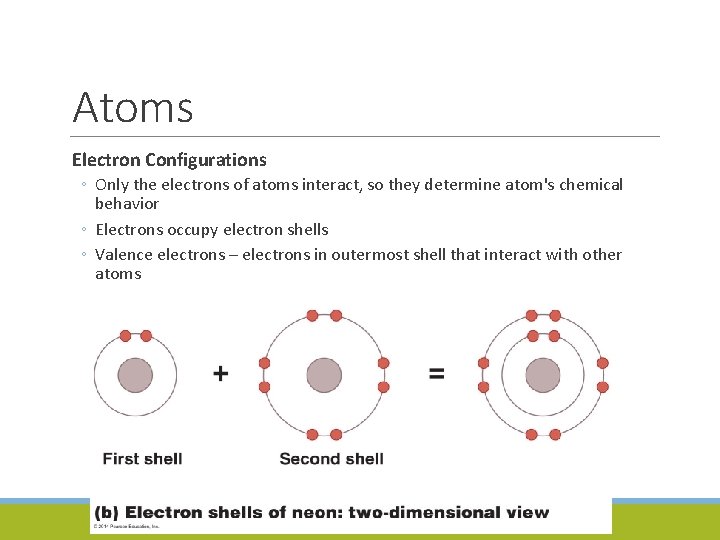
Atoms Electron Configurations ◦ Only the electrons of atoms interact, so they determine atom's chemical behavior ◦ Electrons occupy electron shells ◦ Valence electrons – electrons in outermost shell that interact with other atoms
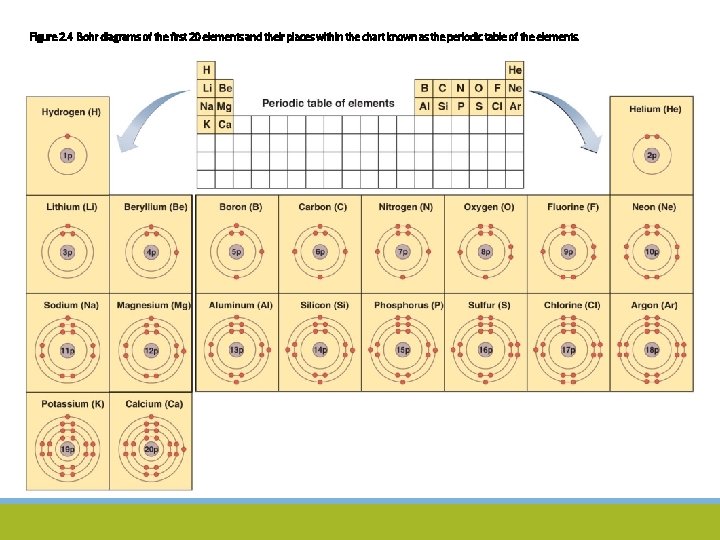
Figure 2. 4 Bohr diagrams of the first 20 elements and their places within the chart known as the periodic table of the elements.
Chemical Bonds Valence – combining capacity of an atom ◦ Positive if atom has electrons to give up ◦ Negative if atom has spaces to fill ◦ Stable when outer electron shells contain eight electrons Chemical bonds – atoms combine by sharing or transferring valence electrons Molecule – two or more atoms held together by chemical bonds Compound – a molecule composed of more than one element
Chemical Bonds Covalent bond – sharing of a pair of electrons by two atoms Electronegativity – attraction of atom for electrons ◦ The more electronegative an atom, the greater the pull its nucleus exerts on electrons
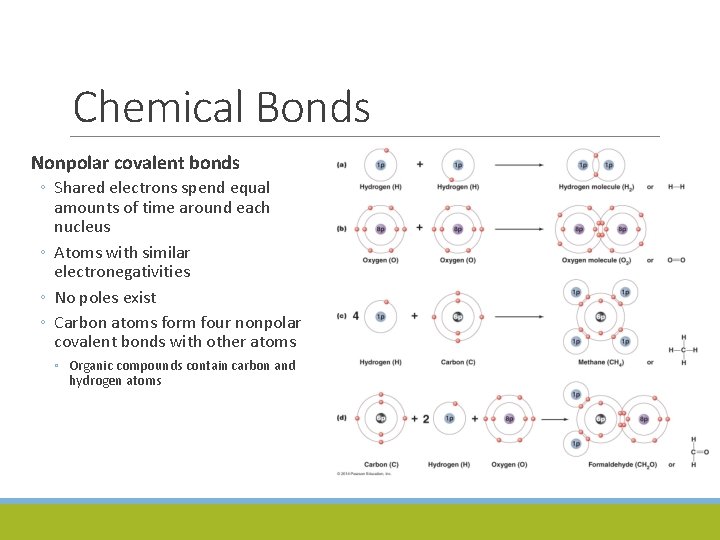
Chemical Bonds Nonpolar covalent bonds ◦ Shared electrons spend equal amounts of time around each nucleus ◦ Atoms with similar electronegativities ◦ No poles exist ◦ Carbon atoms form four nonpolar covalent bonds with other atoms ◦ Organic compounds contain carbon and hydrogen atoms
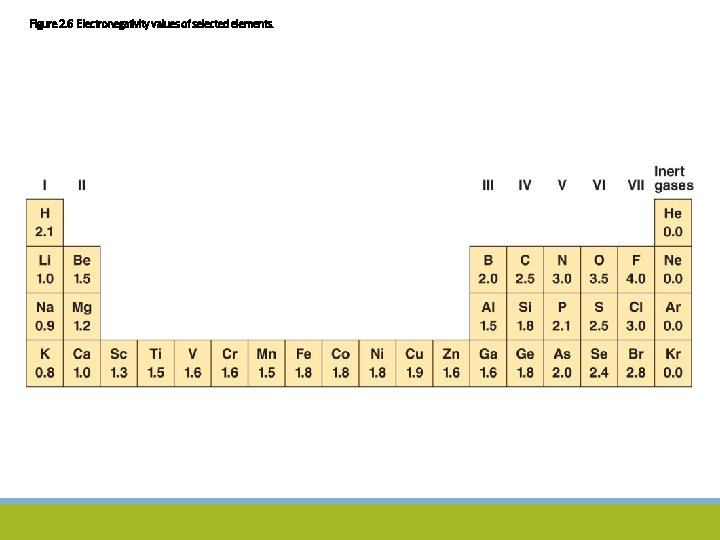
Figure 2. 6 Electronegativity values of selected elements.
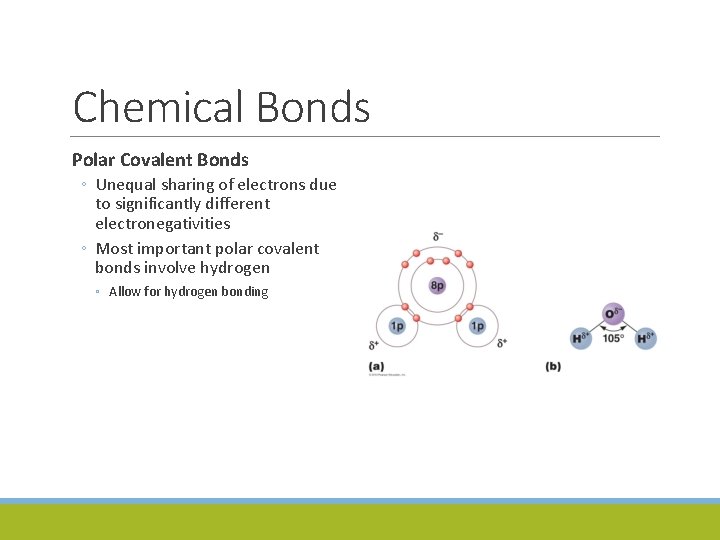
Chemical Bonds Polar Covalent Bonds ◦ Unequal sharing of electrons due to significantly different electronegativities ◦ Most important polar covalent bonds involve hydrogen ◦ Allow for hydrogen bonding
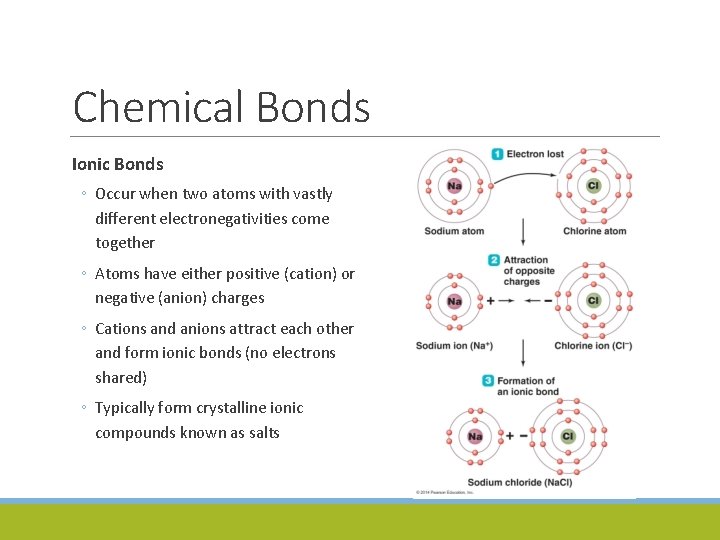
Chemical Bonds Ionic Bonds ◦ Occur when two atoms with vastly different electronegativities come together ◦ Atoms have either positive (cation) or negative (anion) charges ◦ Cations and anions attract each other and form ionic bonds (no electrons shared) ◦ Typically form crystalline ionic compounds known as salts
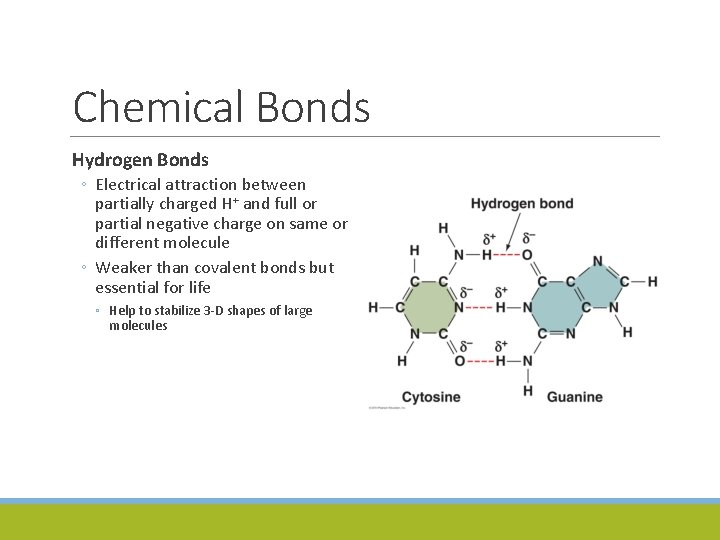
Chemical Bonds Hydrogen Bonds ◦ Electrical attraction between partially charged H+ and full or partial negative charge on same or different molecule ◦ Weaker than covalent bonds but essential for life ◦ Help to stabilize 3 -D shapes of large molecules

Chemical Reactions The making or breaking of chemical bonds Involve reactants and products Biochemistry involves chemical reactions of living things

Chemical Reactions Synthesis Reactions ◦ Involve the formation of larger, more complex molecules ◦ Require energy (endothermic) ◦ Common type is dehydration synthesis ◦ Water molecule formed ◦ All the synthesis reactions in an organism are called anabolism

Chemical Reactions Decomposition Reactions ◦ Break bonds within larger molecules to form smaller atoms, ions, and molecules ◦ Release energy (exothermic) ◦ Common type is hydrolysis ◦ Ionic components of water are added to products ◦ All the decomposition reactions in an organism are called catabolism

Chemical Reactions Exchange Reactions ◦ ◦ Involve breaking and forming covalent bonds Have endothermic and exothermic steps Involve atoms moving from one molecule to another Sum of all chemical reactions in an organism is called metabolism

Water, Acids, Bases, and Salts Water ◦ Most abundant substance in organisms ◦ Many special characteristics due to two polar covalent bonds ◦ Cohesive molecules – surface tension ◦ Excellent solvent ◦ Remains liquid across wide range of temperatures ◦ Can absorb significant amounts of energy without changing temperature ◦ Participates in many chemical reactions
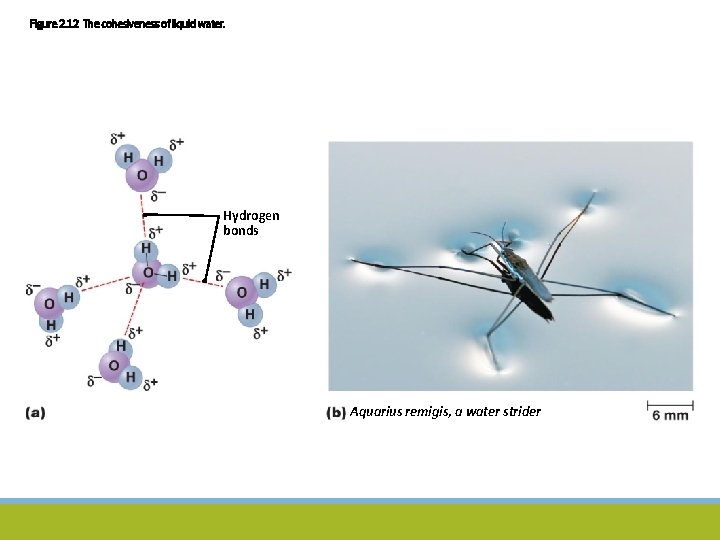
Figure 2. 12 The cohesiveness of liquid water. Hydrogen bonds Aquarius remigis, a water strider

Water, Acids, Bases, and Salts Acids and Bases ◦ Dissociated by water into component cations and anions ◦ Acid – dissociates into one or more H+ and one or more anions ◦ Base – binds with H+ when dissolved into water; some dissociate into cations and OH–
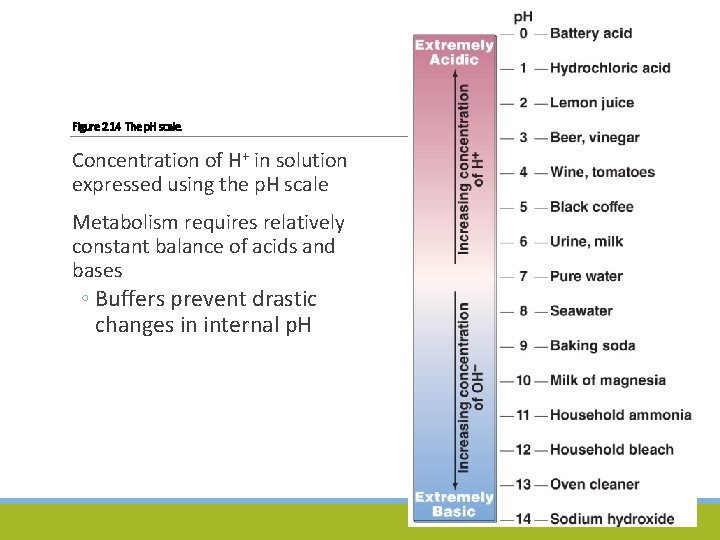
Figure 2. 14 The p. H scale. Concentration of H+ in solution expressed using the p. H scale Metabolism requires relatively constant balance of acids and bases ◦ Buffers prevent drastic changes in internal p. H

Water, Acids, Bases, and Salts ◦ Compounds that dissociate in water into cations and anions other than H+ and OH– ◦ Cations and anions of salts are electrolytes ◦ Create electrical differences between inside and outside of cell ◦ Transfer electrons from one location to another ◦ Form important components of many enzymes
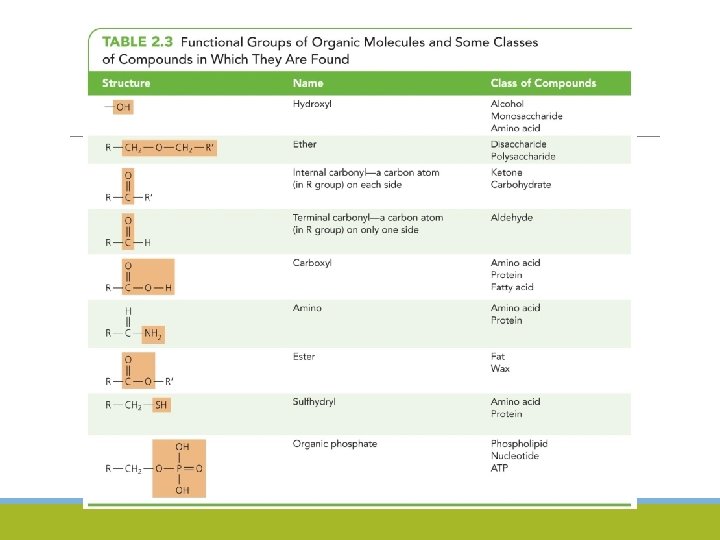
Organic Macromolecules Functional Groups ◦ Contain carbon and hydrogen atoms ◦ Atoms often appear in arrangements called functional groups ◦ Macromolecules – large molecules used by all organisms ◦ Lipids ◦ Carbohydrates ◦ Proteins ◦ Nucleic acids ◦ Monomers – basic building blocks of macromolecules
Organic Macromolecules Lipids ◦ Not composed of regular subunits ◦ Are all hydrophobic ◦ Four groups ◦ ◦ Fats Phospholipids Waxes Steroids
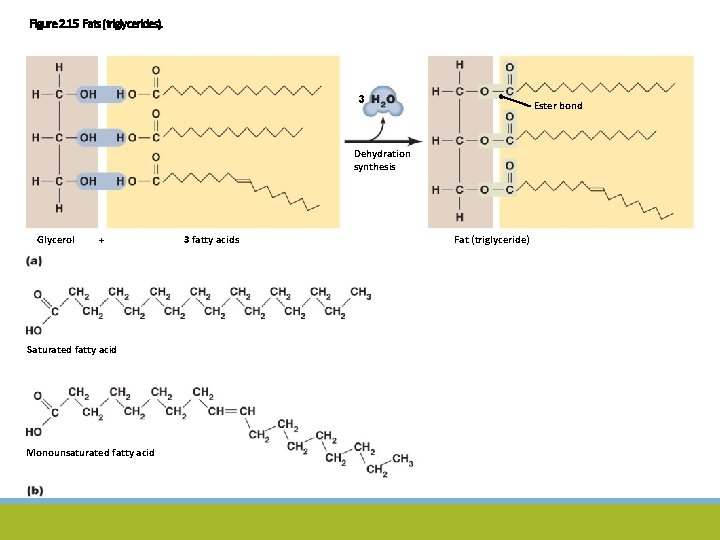
Figure 2. 15 Fats (triglycerides). 3 Ester bond Dehydration synthesis Glycerol + Saturated fatty acid Monounsaturated fatty acid 3 fatty acids Fat (triglyceride)
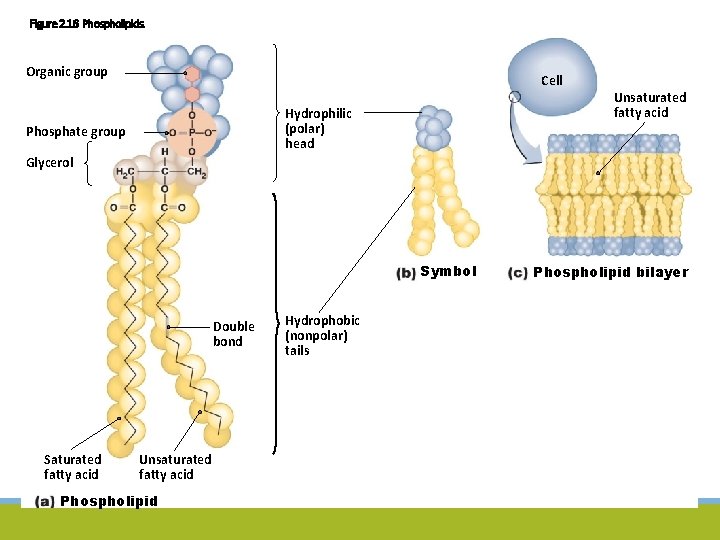
Figure 2. 16 Phospholipids. Organic group Cell Hydrophilic (polar) head Phosphate group Unsaturated fatty acid Glycerol Symbol Double bond Saturated fatty acid Unsaturated fatty acid Phospholipid Hydrophobic (nonpolar) tails Phospholipid bilayer
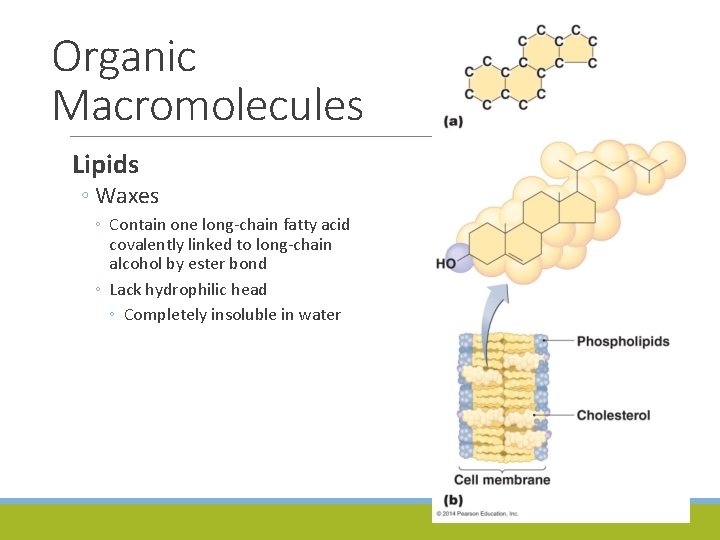
Organic Macromolecules Lipids ◦ Waxes ◦ Contain one long-chain fatty acid covalently linked to long-chain alcohol by ester bond ◦ Lack hydrophilic head ◦ Completely insoluble in water
Organic Macromolecules Carbohydrates ◦ Organic molecules composed of carbon, hydrogen, and oxygen (CH 2 O)n ◦ Functions ◦ Long-term storage of chemical energy ◦ Ready energy source ◦ Part of backbones of nucleic acids ◦ Converted to amino acids ◦ Form cell wall ◦ Involved in intracellular interactions between animal cells
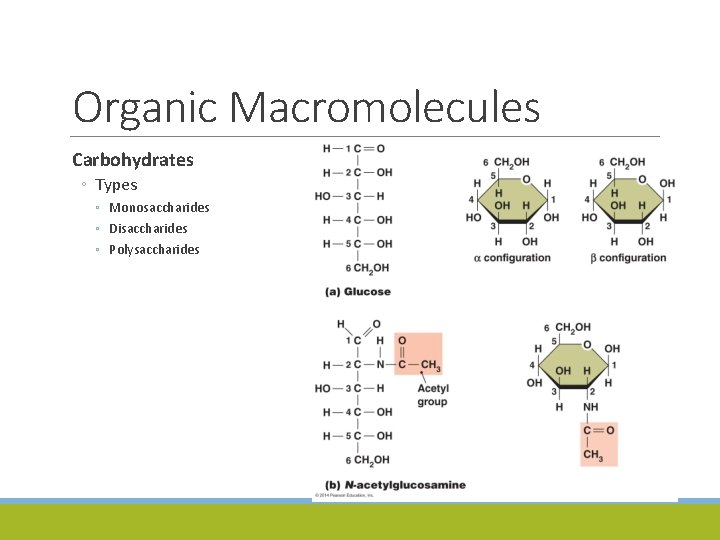
Organic Macromolecules Carbohydrates ◦ Types ◦ Monosaccharides ◦ Disaccharides ◦ Polysaccharides
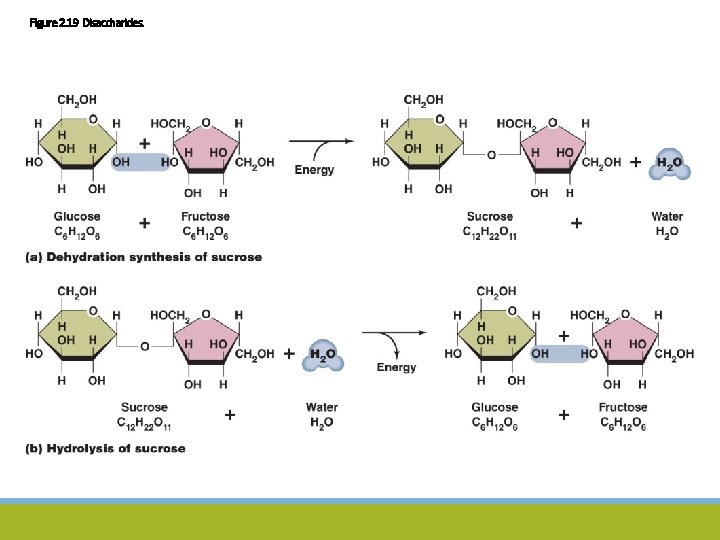
Figure 2. 19 Disaccharides.
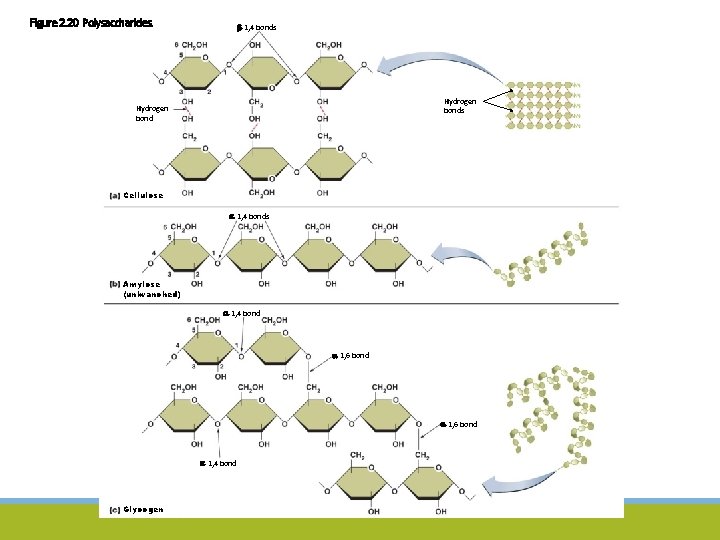
Figure 2. 20 Polysaccharides. -1, 4 bonds Hydrogen bond Cellulose -1, 4 bonds Amylose (unbranched) -1, 4 bond -1, 6 bond -1, 4 bond Glycogen
Organic Macromolecules Proteins ◦ Mostly composed of carbon, hydrogen, oxygen, nitrogen, and sulfur ◦ Functions ◦ ◦ ◦ Structure Enzymatic catalysis Regulation Transportation Defense and offense
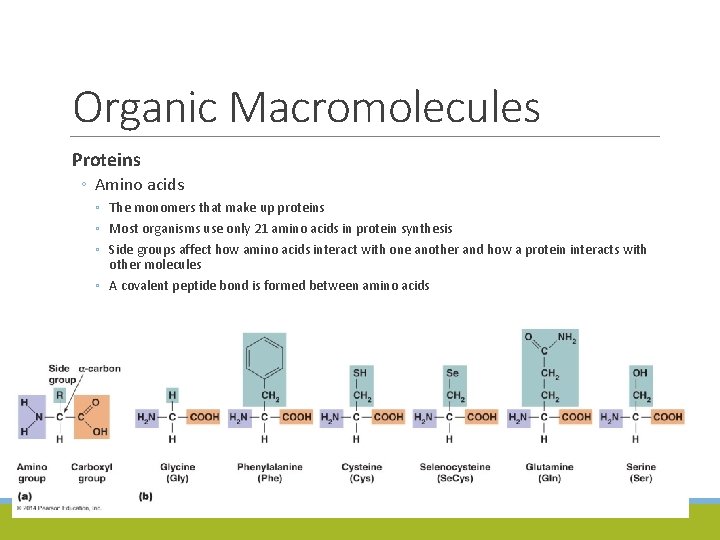
Organic Macromolecules Proteins ◦ Amino acids ◦ The monomers that make up proteins ◦ Most organisms use only 21 amino acids in protein synthesis ◦ Side groups affect how amino acids interact with one another and how a protein interacts with other molecules ◦ A covalent peptide bond is formed between amino acids
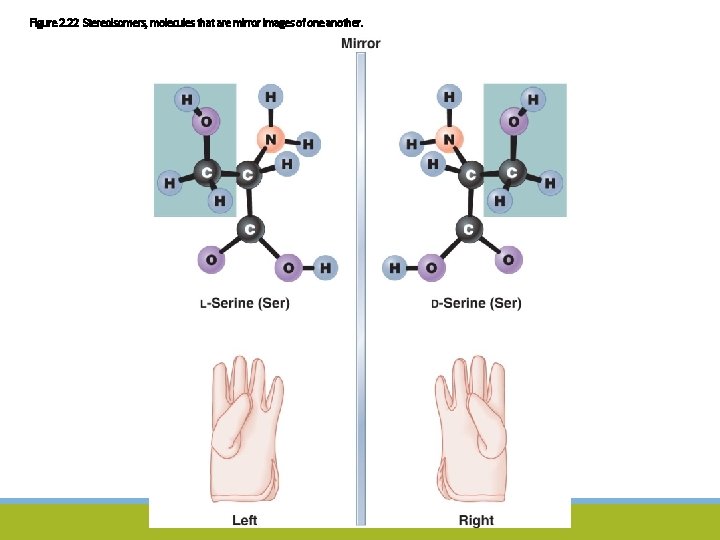
Figure 2. 22 Stereoisomers, molecules that are mirror images of one another.
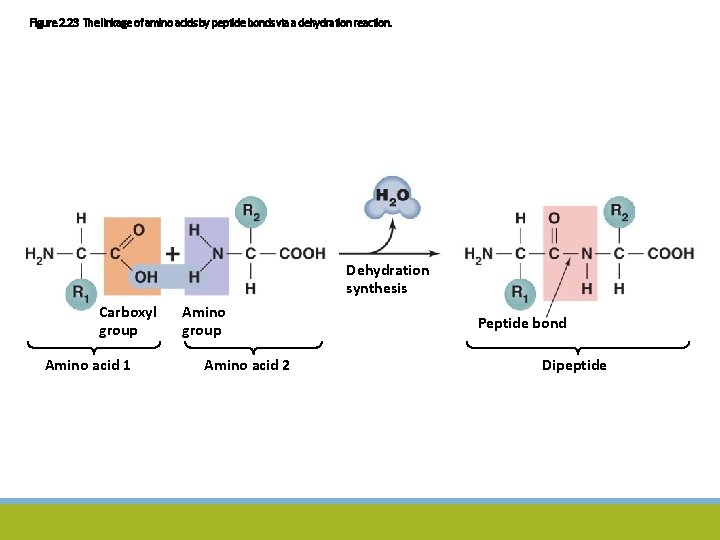
Figure 2. 23 The linkage of amino acids by peptide bonds via a dehydration reaction. Dehydration synthesis Carboxyl group Amino acid 1 Amino group Amino acid 2 Peptide bond Dipeptide
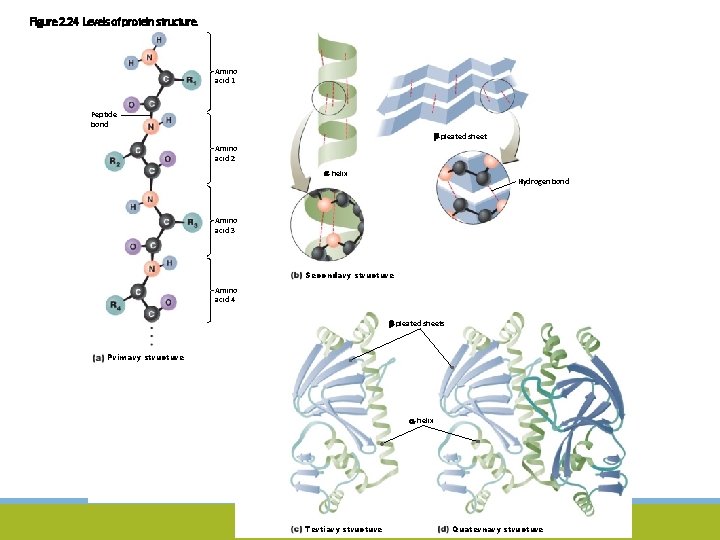
Figure 2. 24 Levels of protein structure. Amino acid 1 Peptide bond -pleated sheet Amino acid 2 -helix Hydrogen bond Amino acid 3 Secondary structure Amino acid 4 -pleated sheets Primary structure -helix Tertiary structure Quaternary structure
Organic Macromolecules Nucleic Acids ◦ DNA and RNA are the genetic material of organisms and viruses ◦ RNA also acts as enzyme, binds amino acids, and helps form polypeptides
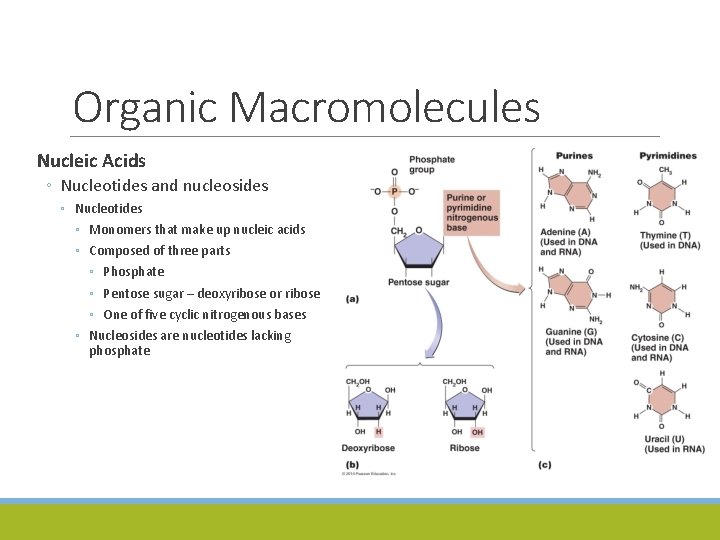
Organic Macromolecules Nucleic Acids ◦ Nucleotides and nucleosides ◦ Nucleotides ◦ Monomers that make up nucleic acids ◦ Composed of three parts ◦ Phosphate ◦ Pentose sugar – deoxyribose or ribose ◦ One of five cyclic nitrogenous bases ◦ Nucleosides are nucleotides lacking phosphate
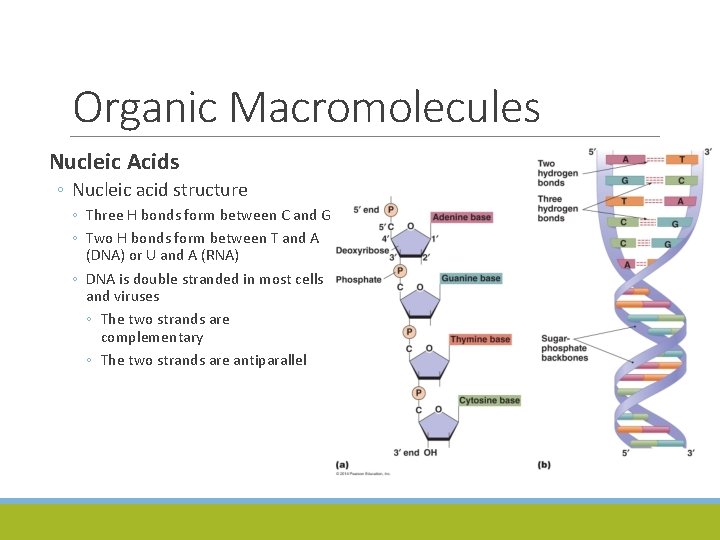
Organic Macromolecules Nucleic Acids ◦ Nucleic acid structure ◦ Three H bonds form between C and G ◦ Two H bonds form between T and A (DNA) or U and A (RNA) ◦ DNA is double stranded in most cells and viruses ◦ The two strands are complementary ◦ The two strands are antiparallel
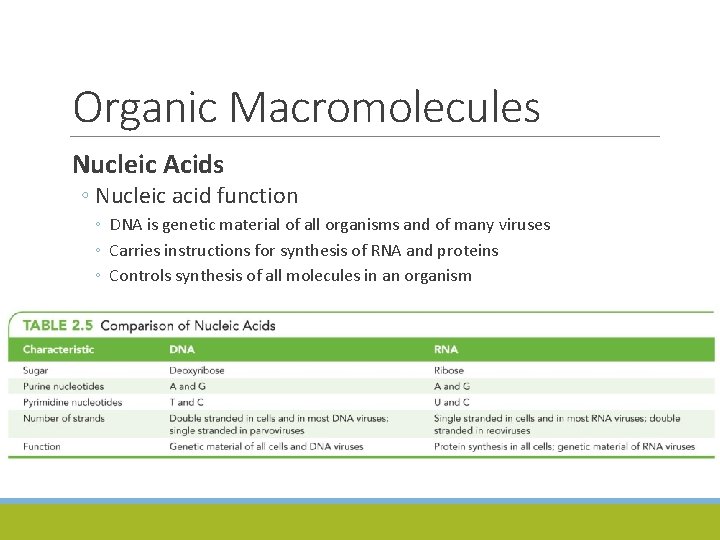
Organic Macromolecules Nucleic Acids ◦ Nucleic acid function ◦ DNA is genetic material of all organisms and of many viruses ◦ Carries instructions for synthesis of RNA and proteins ◦ Controls synthesis of all molecules in an organism
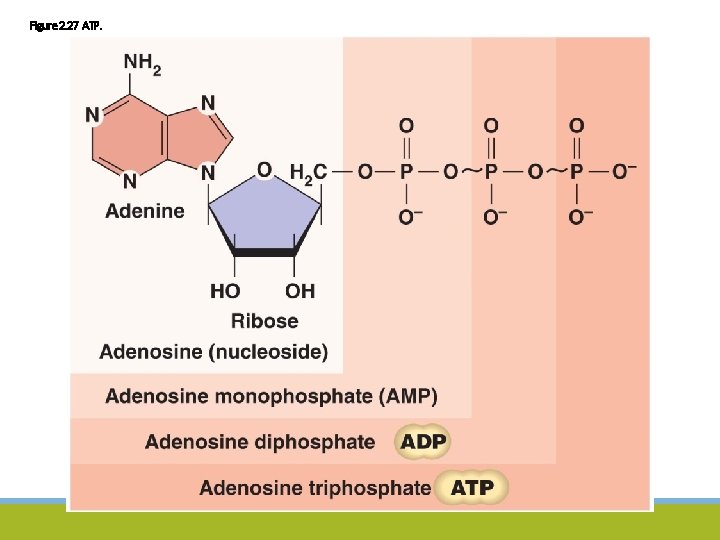
Figure 2. 27 ATP.
- Slides: 43