The Chemistry of Life The Properties of Water






- Slides: 25

The Chemistry of Life The Properties of Water

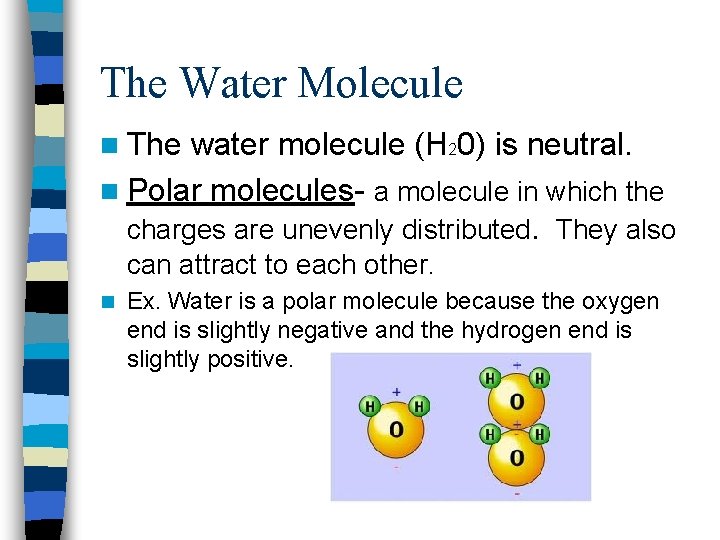
The Water Molecule n The water molecule (H 20) is neutral. n Polar molecules- a molecule in which the charges are unevenly distributed. They also can attract to each other. n Ex. Water is a polar molecule because the oxygen end is slightly negative and the hydrogen end is slightly positive.

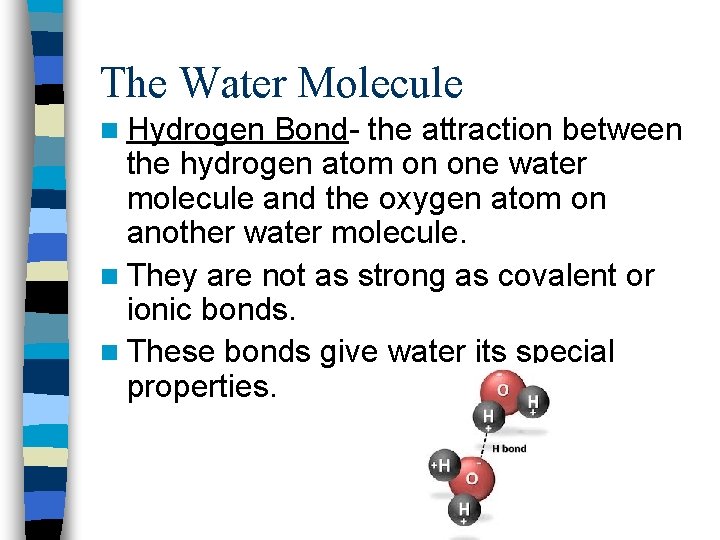
The Water Molecule n Hydrogen Bond- the attraction between the hydrogen atom on one water molecule and the oxygen atom on another water molecule. n They are not as strong as covalent or ionic bonds. n These bonds give water its special properties.

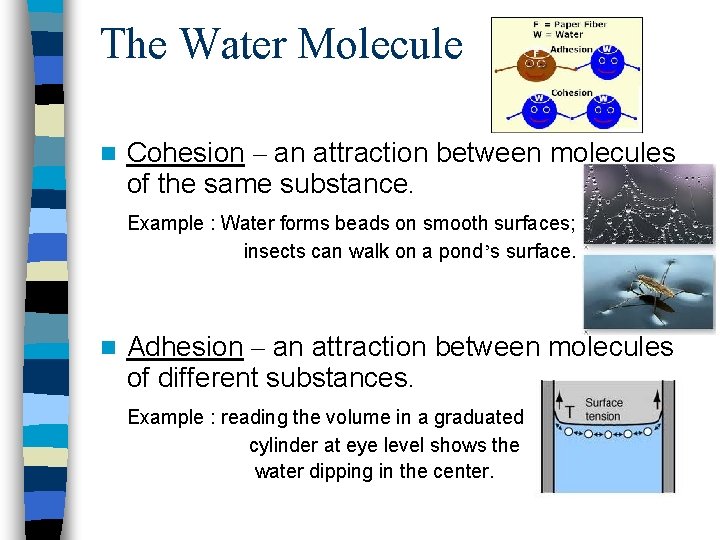
The Water Molecule n Cohesion – an attraction between molecules of the same substance. Example : Water forms beads on smooth surfaces; insects can walk on a pond’s surface. n Adhesion – an attraction between molecules of different substances. Example : reading the volume in a graduated cylinder at eye level shows the water dipping in the center.

Solutions and Suspensions n Mixture – a material composed of two or more elements or compounds that are physically mixed together but not chemically combined. Example: Stirring together salt/pepper, sugar/sand.

Solutions It is a mixture in which all the components are evenly distributed throughout the mixture. n Table salt in warm water – the sodium and the chloride ions are attracted to the polar water molecules thus the salt dissolves into the water. n Solute – substance that is dissolved. Ex. salt n Solvent – substance in which the solute dissolves. Ex. water n

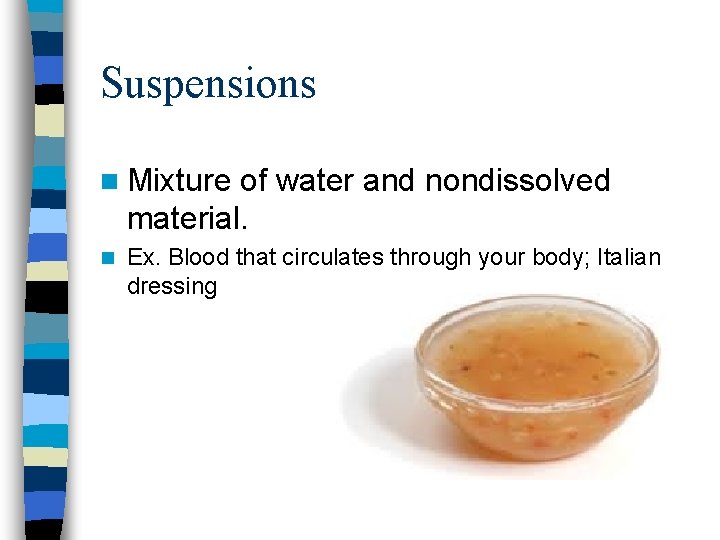
Suspensions n Mixture of water and nondissolved material. n Ex. Blood that circulates through your body; Italian dressing

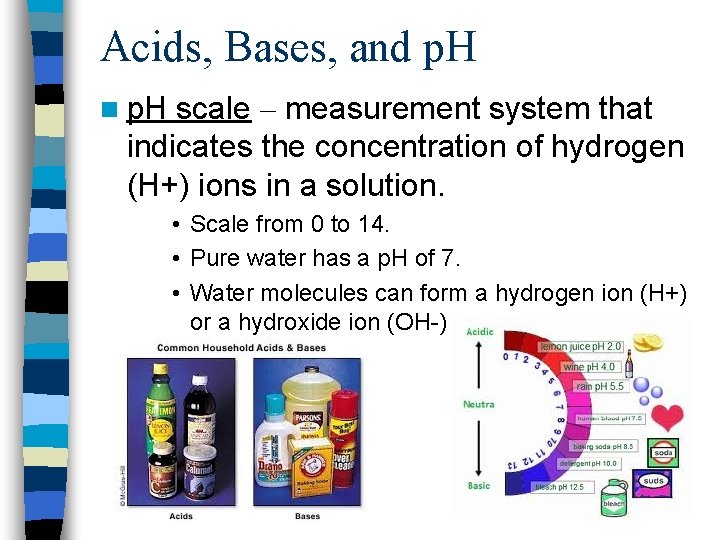
Acids, Bases, and p. H n p. H scale – measurement system that indicates the concentration of hydrogen (H+) ions in a solution. • Scale from 0 to 14. • Pure water has a p. H of 7. • Water molecules can form a hydrogen ion (H+) or a hydroxide ion (OH-)

Acids, Bases, and p. H n Acids – any compound that forms H+ ions in a solution. • The lower end (below 7) of a p. H scale. • Has a higher concentration of H+ ions than OHor pure water. • The lower the p. H the greater the acidity. • Ex. -Stomach acid, lemon juice, tomato juice, acid rain.

Acids, Bases, and p. H n Bases – a compound that produces OHions in a solution. Alkaline • The higher end (above 7) of a p. H scale. • Has a lower concentration of H+ ions than OHor pure water. • The higher the p. H the stronger the base. • Ex. -oven cleaner, bleach, ammonia solutions, soap.

Carbon Compounds The Chemistry of Carbon

Organic Chemistry n The study of all compounds that contain bonds between carbon atoms. n Carbon compounds are also called organic compounds.

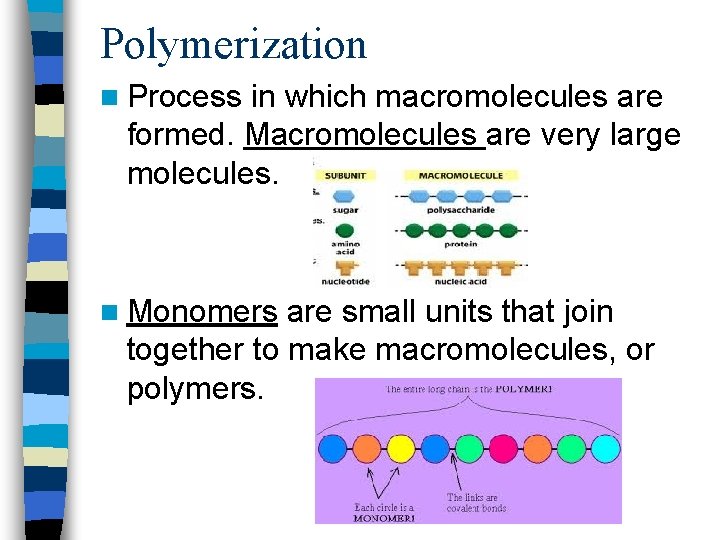
Polymerization n Process in which macromolecules are formed. Macromolecules are very large molecules. n Monomers are small units that join together to make macromolecules, or polymers.
4 groups of organic compounds found in living things: n Carbohydrates n Lipids n Nucleic acids n Proteins

Carbohydrates n Made up of carbon, hydrogen, and oxygen atoms. n Living things use it as their main source of energy. n Living things store sugar as complex carbohydrates known as starches. n Ex. pasta

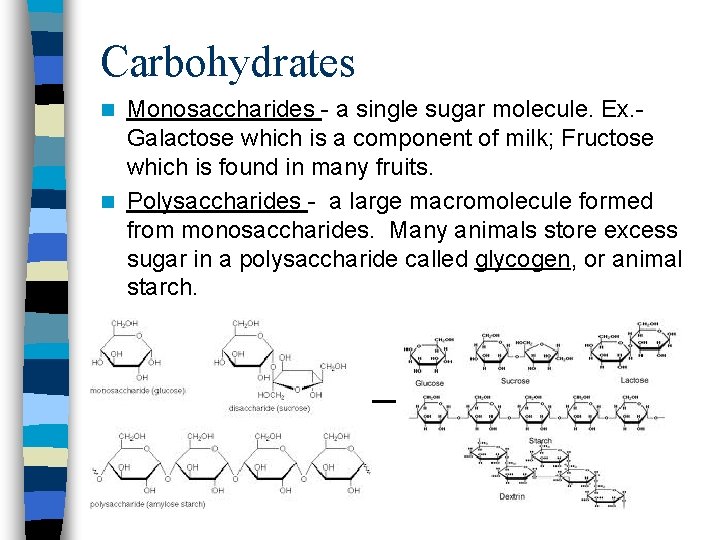
Carbohydrates Monosaccharides - a single sugar molecule. Ex. Galactose which is a component of milk; Fructose which is found in many fruits. n Polysaccharides - a large macromolecule formed from monosaccharides. Many animals store excess sugar in a polysaccharide called glycogen, or animal starch. n

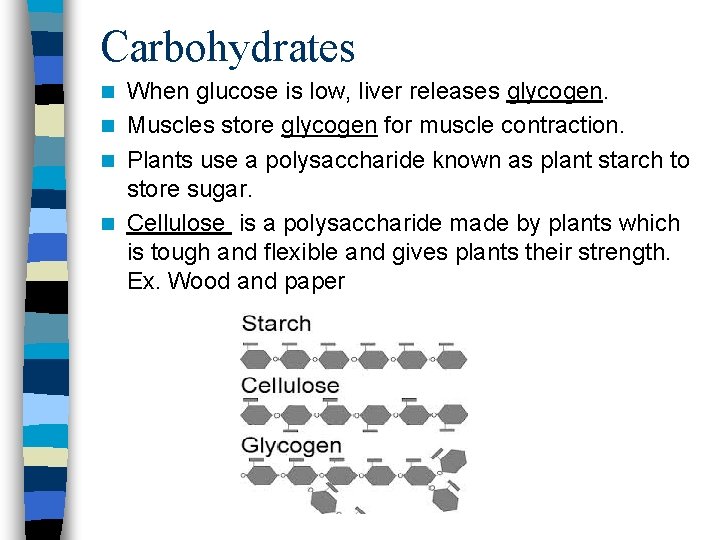
Carbohydrates When glucose is low, liver releases glycogen. n Muscles store glycogen for muscle contraction. n Plants use a polysaccharide known as plant starch to store sugar. n Cellulose is a polysaccharide made by plants which is tough and flexible and gives plants their strength. Ex. Wood and paper n

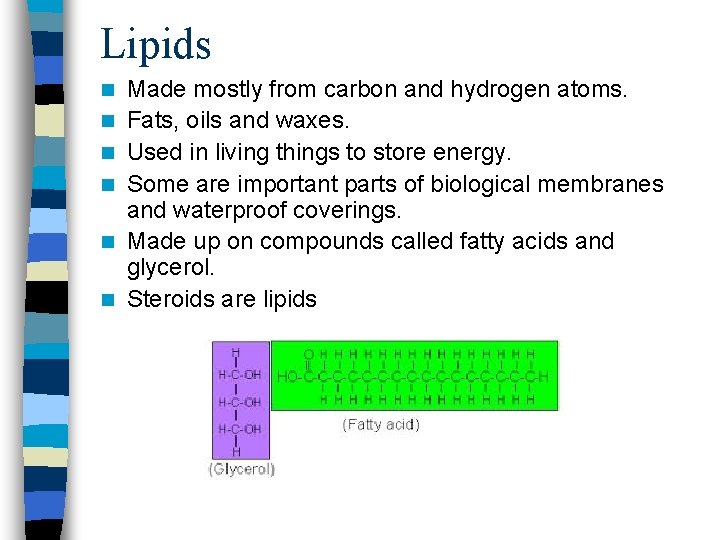
Lipids n n n Made mostly from carbon and hydrogen atoms. Fats, oils and waxes. Used in living things to store energy. Some are important parts of biological membranes and waterproof coverings. Made up on compounds called fatty acids and glycerol. Steroids are lipids

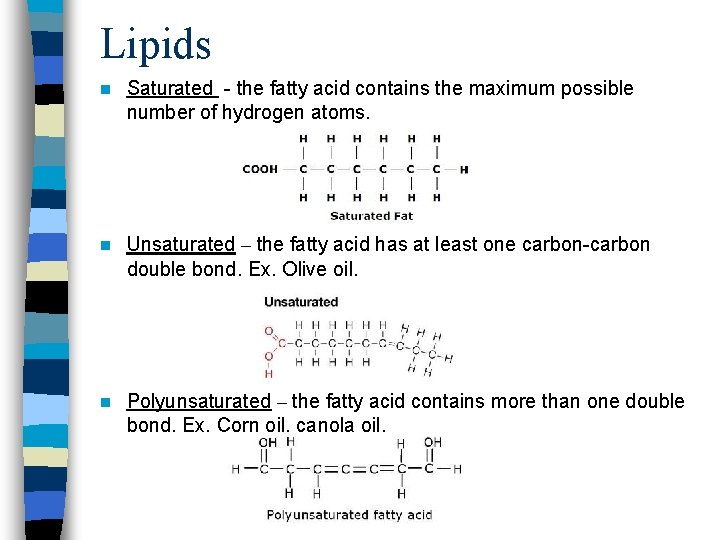
Lipids n Saturated - the fatty acid contains the maximum possible number of hydrogen atoms. n Unsaturated – the fatty acid has at least one carbon-carbon double bond. Ex. Olive oil. n Polyunsaturated – the fatty acid contains more than one double bond. Ex. Corn oil, canola oil.

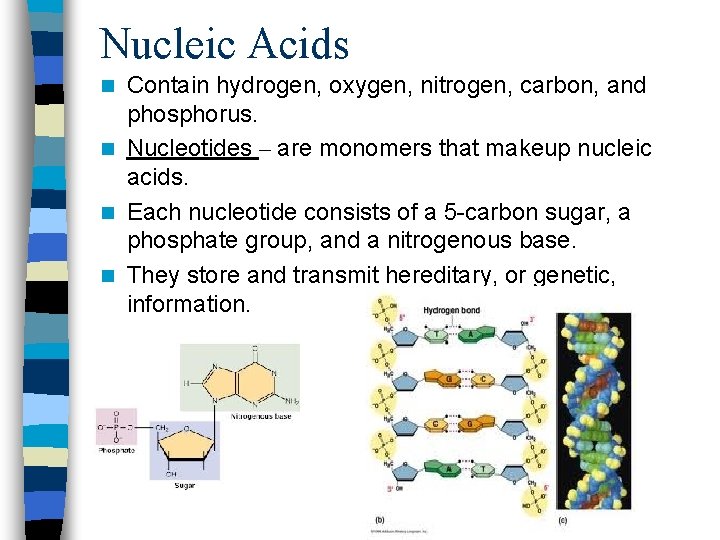
Nucleic Acids Contain hydrogen, oxygen, nitrogen, carbon, and phosphorus. n Nucleotides – are monomers that makeup nucleic acids. n Each nucleotide consists of a 5 -carbon sugar, a phosphate group, and a nitrogenous base. n They store and transmit hereditary, or genetic, information. n

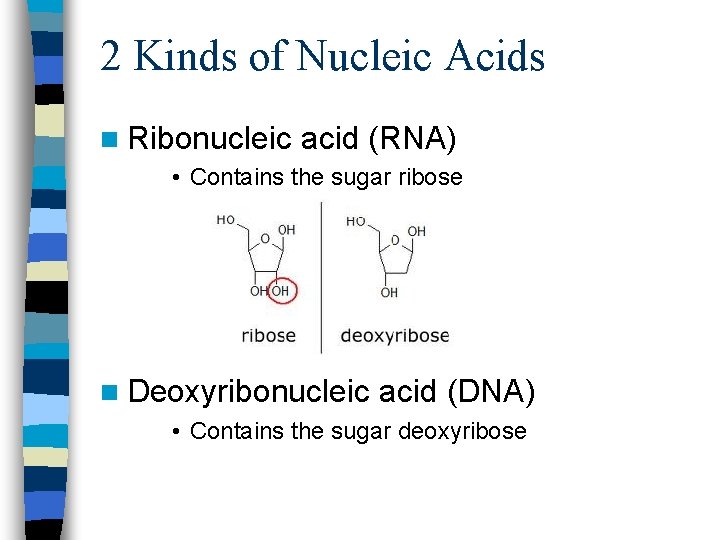
2 Kinds of Nucleic Acids n Ribonucleic acid (RNA) • Contains the sugar ribose n Deoxyribonucleic acid (DNA) • Contains the sugar deoxyribose

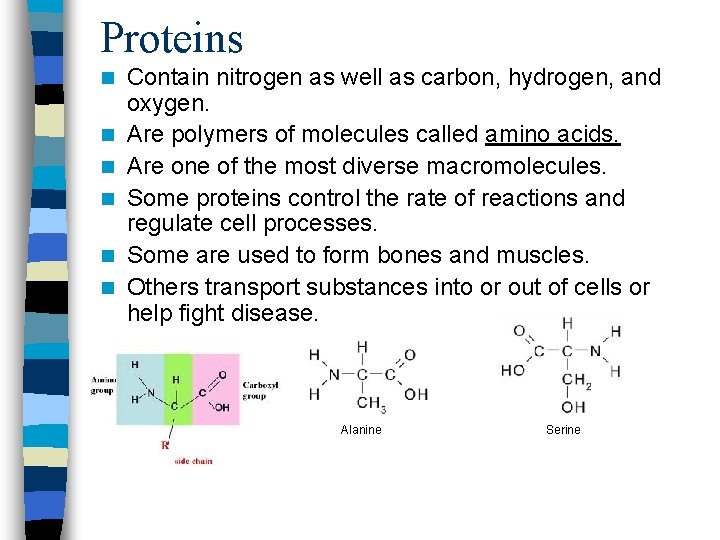
Proteins n n n Contain nitrogen as well as carbon, hydrogen, and oxygen. Are polymers of molecules called amino acids. Are one of the most diverse macromolecules. Some proteins control the rate of reactions and regulate cell processes. Some are used to form bones and muscles. Others transport substances into or out of cells or help fight disease. » Alanine Serine

Chemical Reactions & Enzymes Chemical Reaction – a process that changes one set of chemicals (reactants) into another set of chemicals (products). n Chemical Reactions - always involve the breaking of bonds in reactants and the formation of new bonds in products. n Some chemical reactions release energy (often spontaneously), and others absorb energy. n

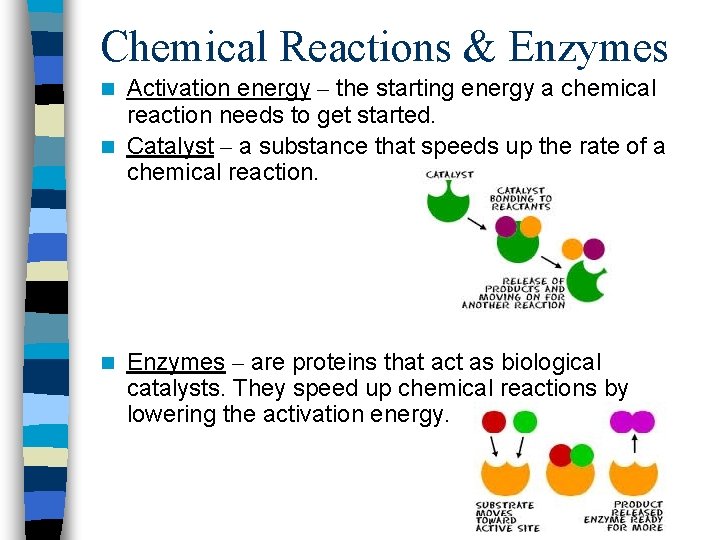
Chemical Reactions & Enzymes Activation energy – the starting energy a chemical reaction needs to get started. n Catalyst – a substance that speeds up the rate of a chemical reaction. n n Enzymes – are proteins that act as biological catalysts. They speed up chemical reactions by lowering the activation energy.

Regulation of Enzyme Activity n Enzymes that help digest food work best at certain p. H values. n Many are affected by changes in temperatures. Temps close to 37 degrees C (normal body temp. )