The Chemistry of Life Organic Chemistry and the





- Slides: 19


The Chemistry of Life Organic Chemistry and the Importance of Carbon

Organic Chemistry • Study of carbon-based compounds • Range from simple molecules (monomers) to large biomolecules (polymers)

Carbon • Very unique in its ability to form complex, diverse molecules • • • large, Has 4 valence electrons Can form up to 4 covalent bonds (tetracovalence) Bonds can be single, double, or triple covalent Molecules can be chains, ring-shaped, or branched Can create many isomers (molecules with same formula, but different atom arrangement)

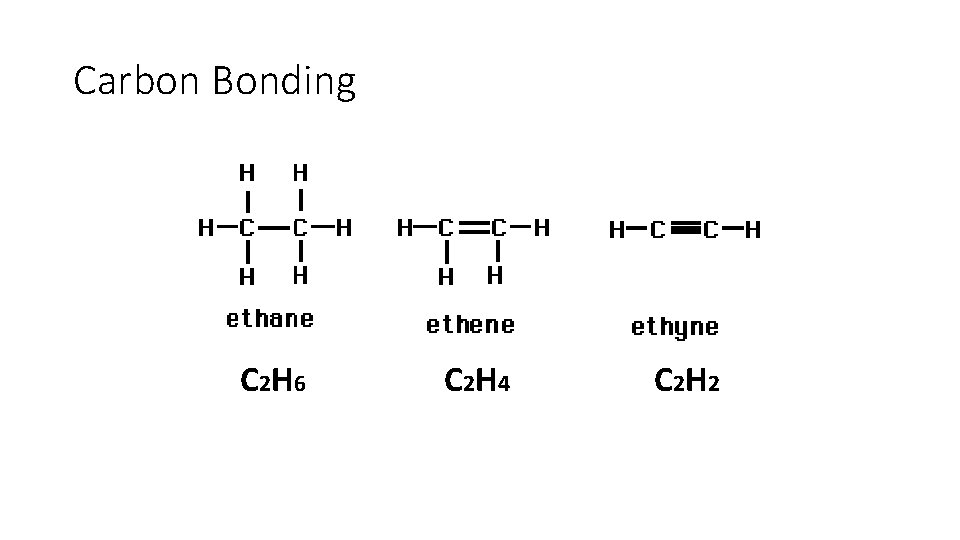
Carbon Bonding C 2 H 6 C 2 H 4 C 2 H 2

Molecule Shape branched linear ring

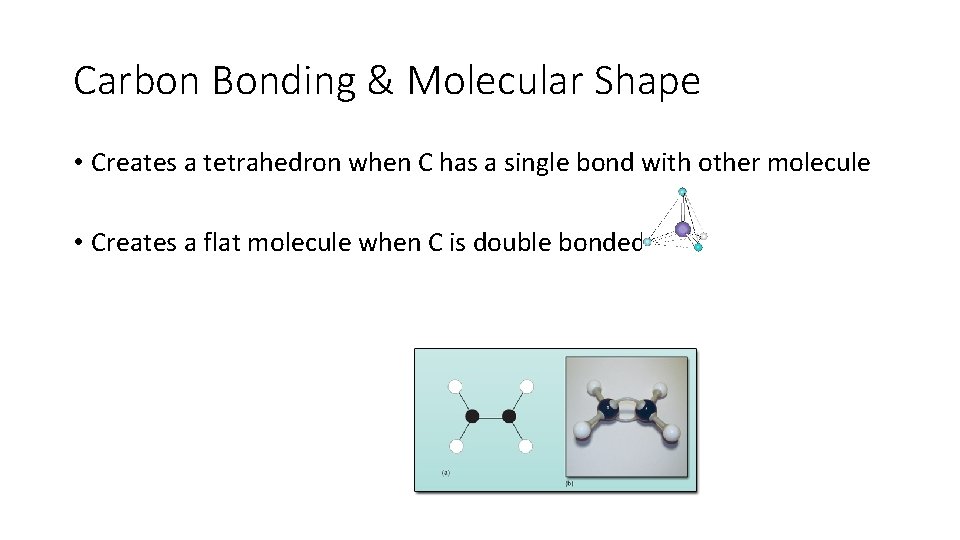
Carbon Bonding & Molecular Shape • Creates a tetrahedron when C has a single bond with other molecule • Creates a flat molecule when C is double bonded

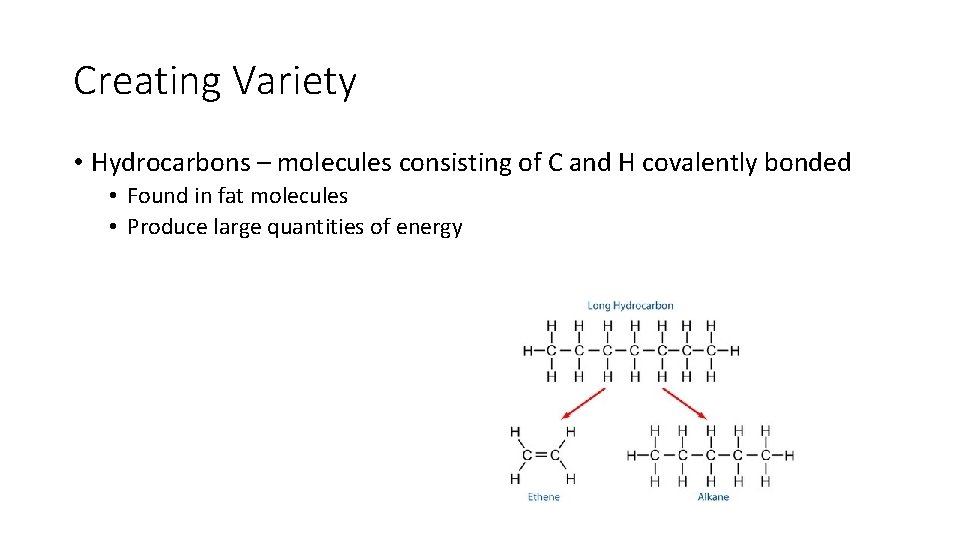
Creating Variety • Hydrocarbons – molecules consisting of C and H covalently bonded • Found in fat molecules • Produce large quantities of energy

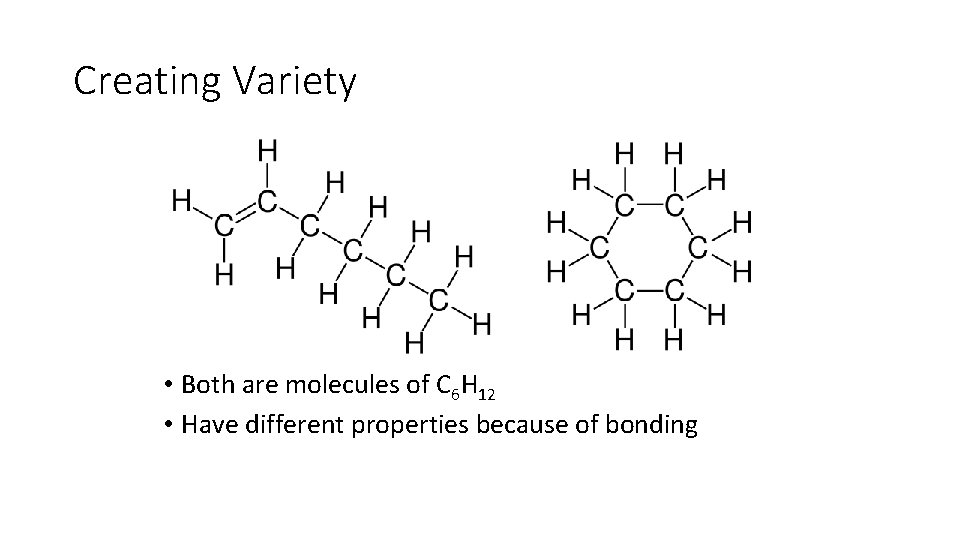
Creating Variety • Both are molecules of C 6 H 12 • Have different properties because of bonding

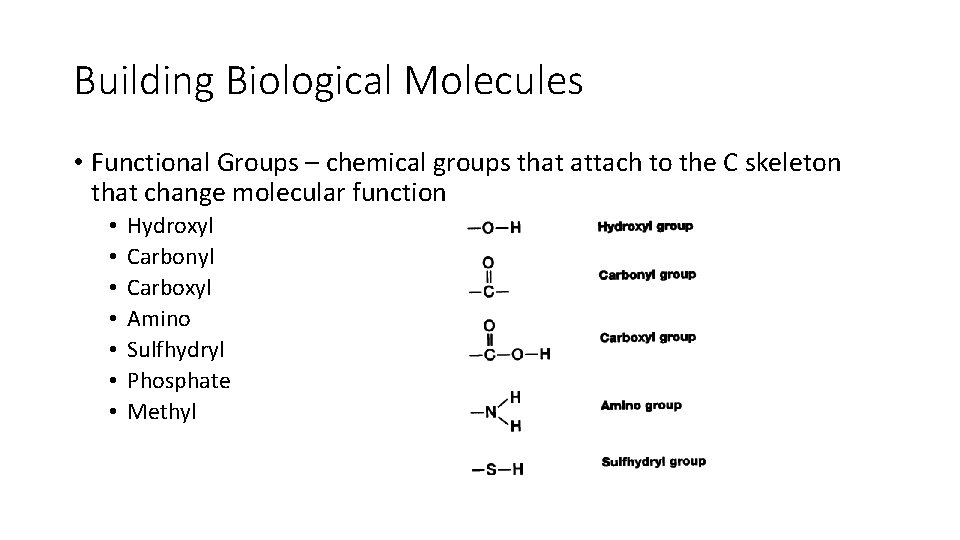
Building Biological Molecules • Functional Groups – chemical groups that attach to the C skeleton that change molecular function • • Hydroxyl Carbonyl Carboxyl Amino Sulfhydryl Phosphate Methyl

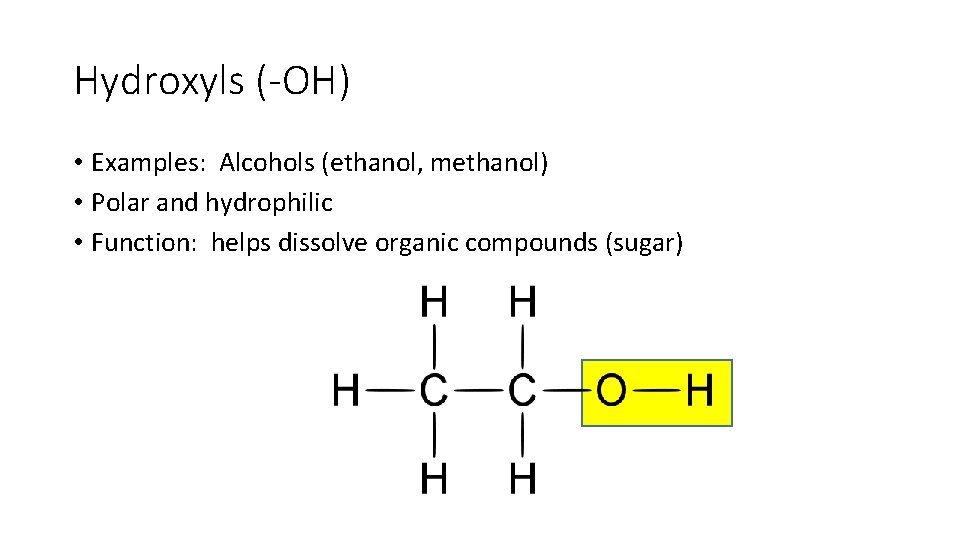
Hydroxyls (-OH) • Examples: Alcohols (ethanol, methanol) • Polar and hydrophilic • Function: helps dissolve organic compounds (sugar)

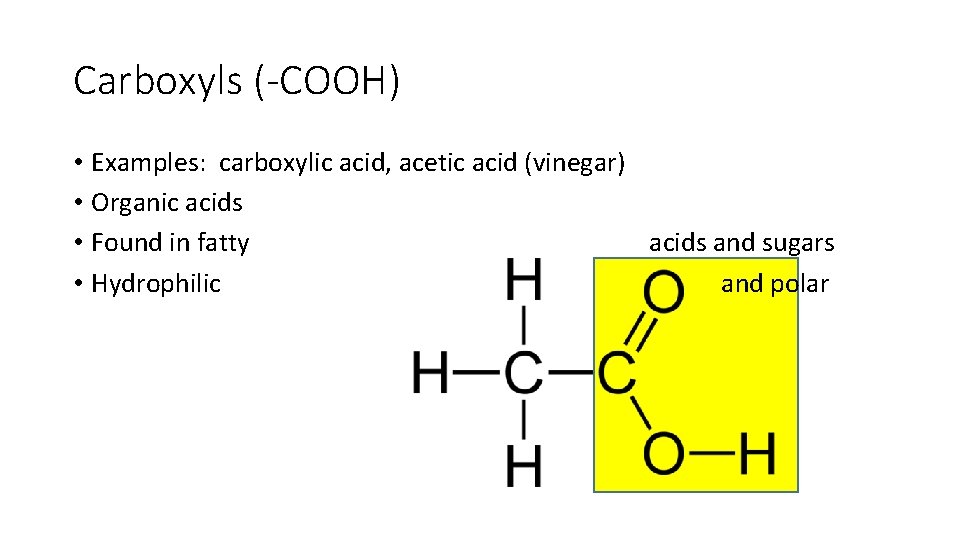
Carboxyls (-COOH) • Examples: carboxylic acid, acetic acid (vinegar) • Organic acids • Found in fatty acids and sugars • Hydrophilic and polar

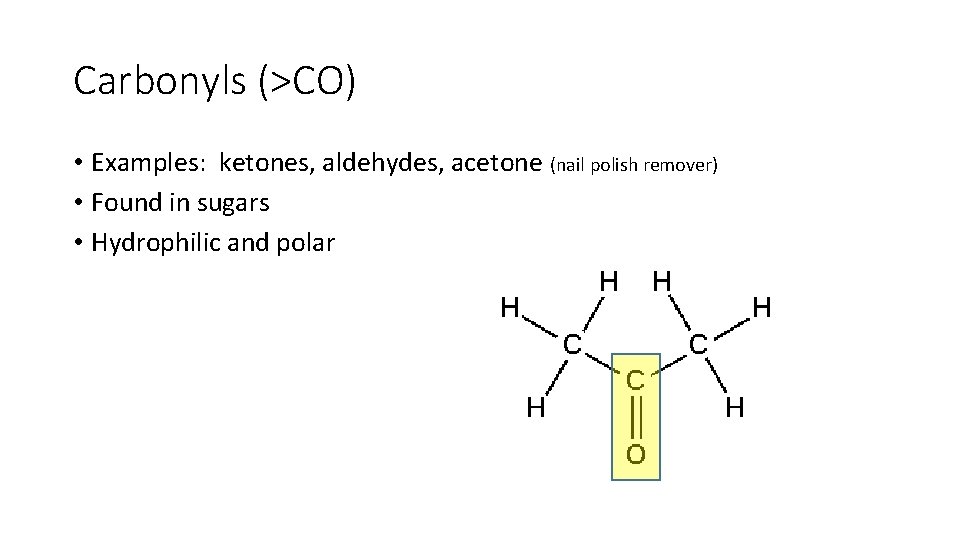
Carbonyls (>CO) • Examples: ketones, aldehydes, acetone (nail polish remover) • Found in sugars • Hydrophilic and polar

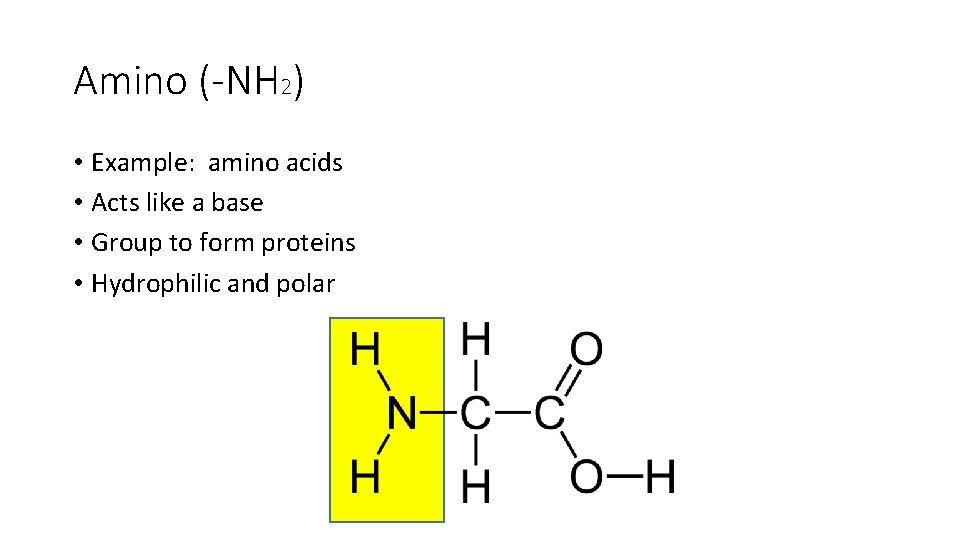
Amino (-NH 2) • Example: amino acids • Acts like a base • Group to form proteins • Hydrophilic and polar

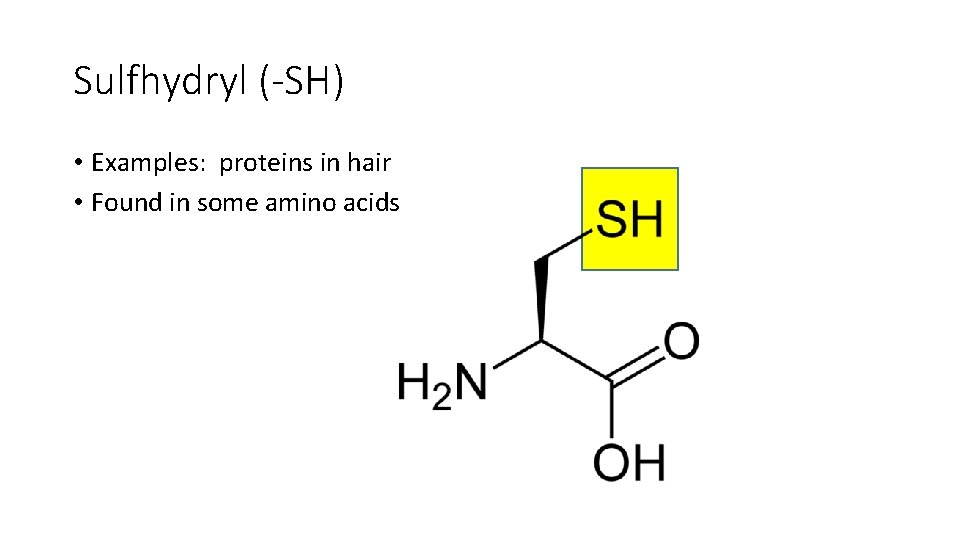
Sulfhydryl (-SH) • Examples: proteins in hair • Found in some amino acids

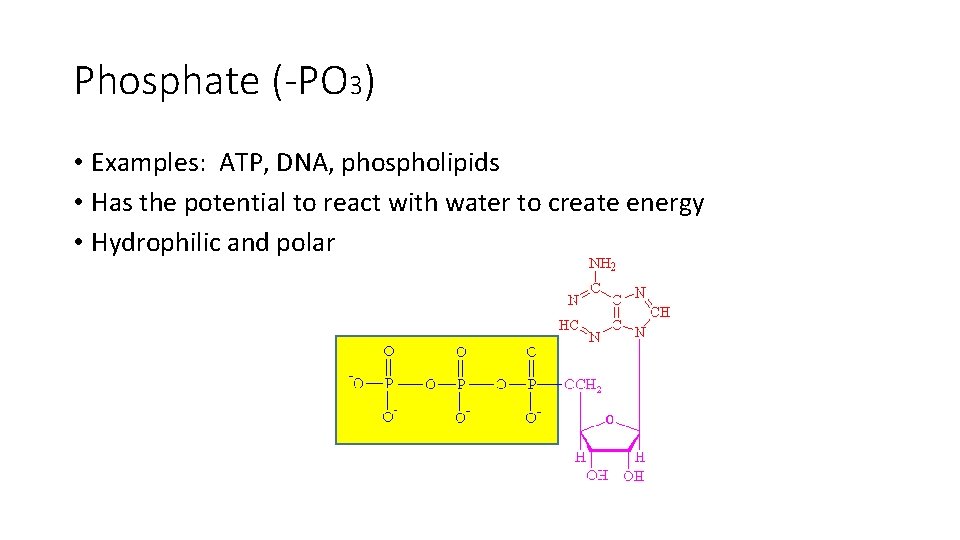
Phosphate (-PO 3) • Examples: ATP, DNA, phospholipids • Has the potential to react with water to create energy • Hydrophilic and polar

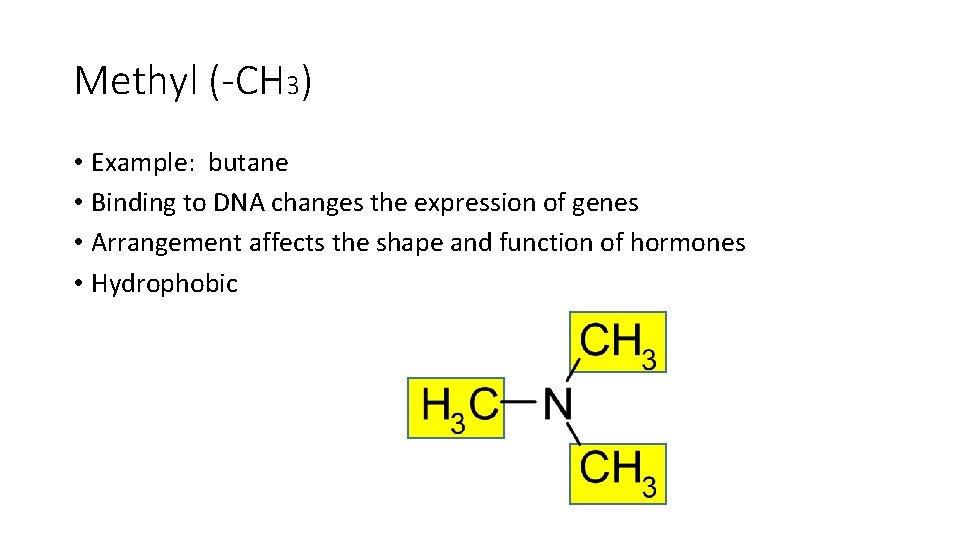
Methyl (-CH 3) • Example: butane • Binding to DNA changes the expression of genes • Arrangement affects the shape and function of hormones • Hydrophobic

