The Chemistry of Life Elements A substance that
The Chemistry of Life

Elements • A substance that can not be broken down into simpler chemical substances. • 90 Natural occurring. • 25 essential for living organisms.

• 4 elements make up 96% of a human body. • carbon, hydrogen, oxygen and nitrogen. • See table 6. 1 page 146.
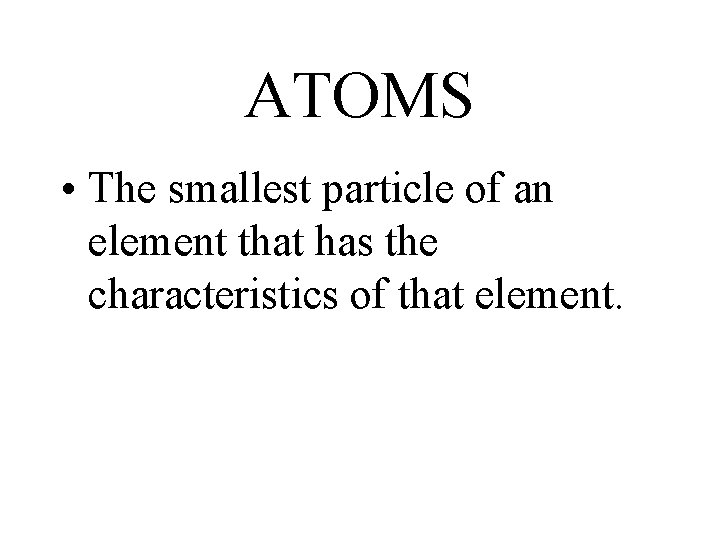
ATOMS • The smallest particle of an element that has the characteristics of that element.
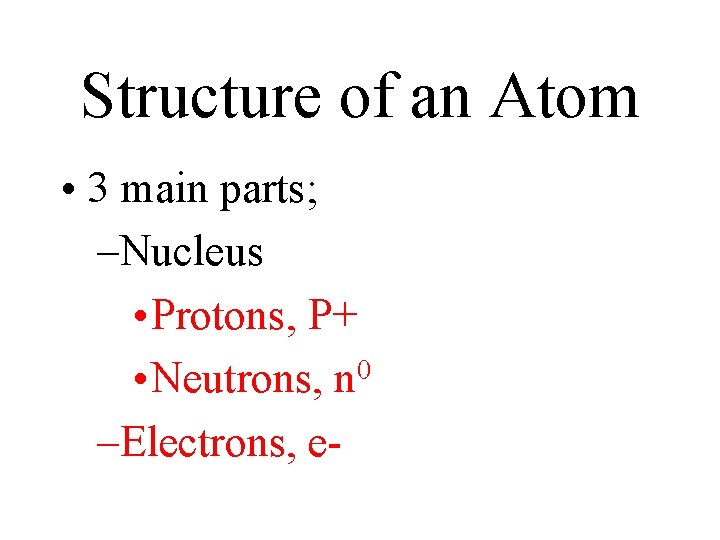
Structure of an Atom • 3 main parts; –Nucleus • Protons, P+ 0 • Neutrons, n –Electrons, e-
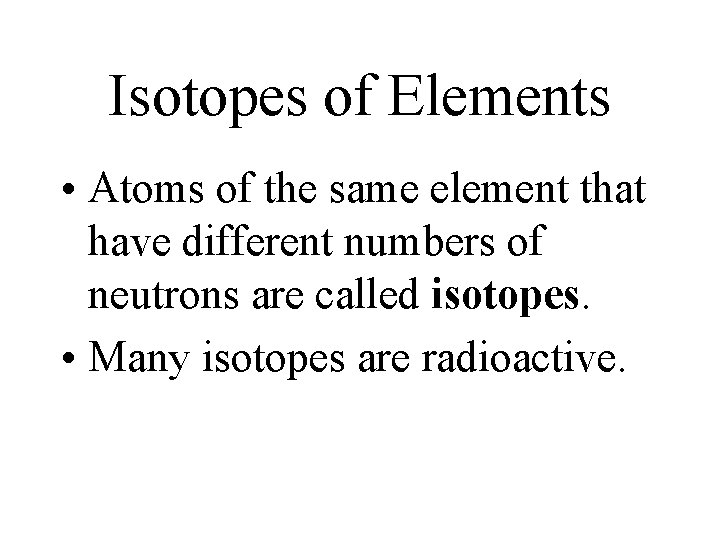
Isotopes of Elements • Atoms of the same element that have different numbers of neutrons are called isotopes. • Many isotopes are radioactive.

Compound • A substance that is composed of atoms of two or more different elements that are chemically combined.
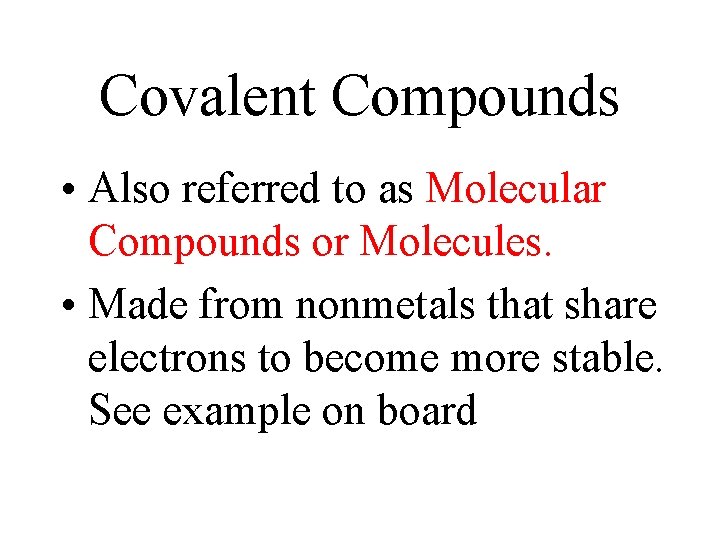
Covalent Compounds • Also referred to as Molecular Compounds or Molecules. • Made from nonmetals that share electrons to become more stable. See example on board
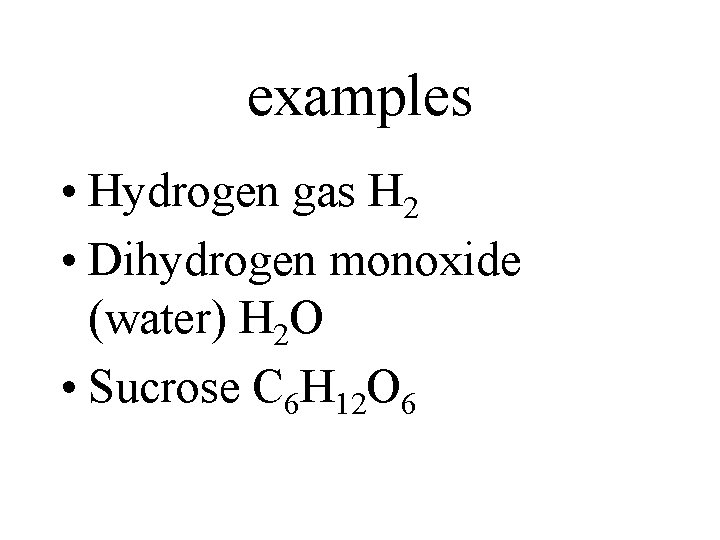
examples • Hydrogen gas H 2 • Dihydrogen monoxide (water) H 2 O • Sucrose C 6 H 12 O 6
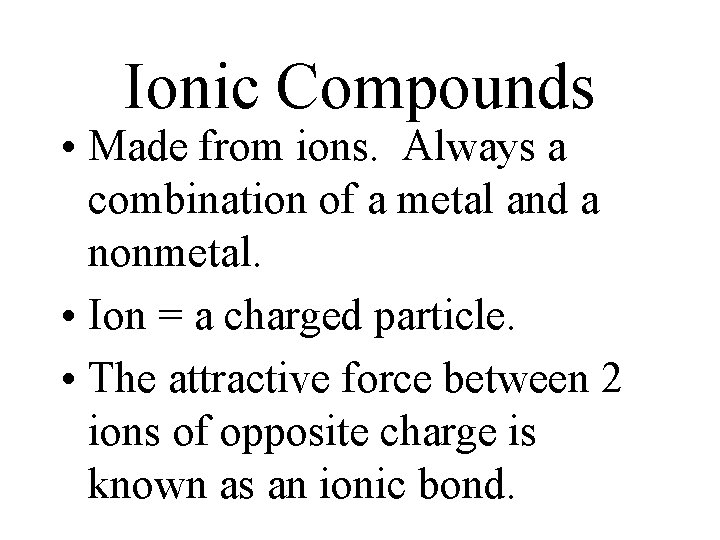
Ionic Compounds • Made from ions. Always a combination of a metal and a nonmetal. • Ion = a charged particle. • The attractive force between 2 ions of opposite charge is known as an ionic bond.
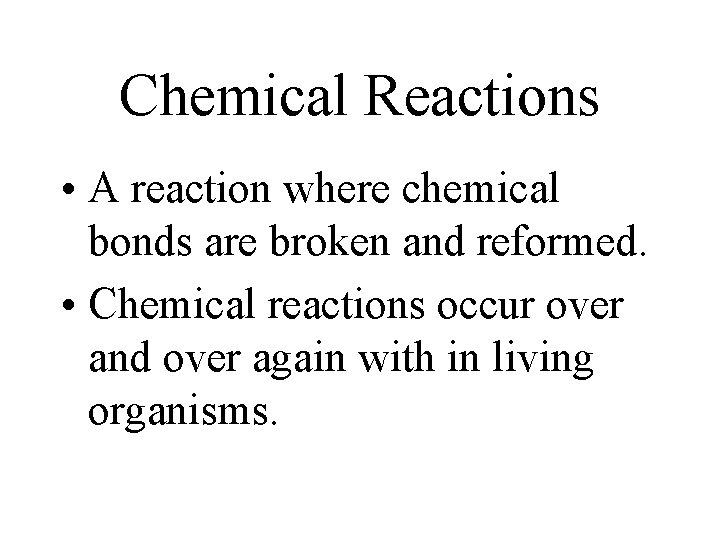
Chemical Reactions • A reaction where chemical bonds are broken and reformed. • Chemical reactions occur over and over again with in living organisms.

Metabolism • All the chemical reactions that occur within an organism.

Mixture • A combination of substances in which the individual components retain their own properties.

Solution • A mixture in which one or more substances are distributed evenly in another substance.
Acids and Bases • p. H is a measure of how acidic or basic a solution is. • The p. H scale is from 0 -14.

ACID • Any substance that forms hydrogen ions when dissolved in water. • p. H below 7 = ACIDIC

BASE • Any substance that forms hydroxide ions when dissolved in water. • p. H above 7 = BASIC

ASSIGNMENT • SA 6. 1, #1 -6 page 155.

WATER • Water serves as a means of material transportation in organisms. • Most organisms are composed of between 70 to 95% water.

Water is Polar • A polar molecule is a molecule with an unequal distribution of charge.

Because Water is Polar. . . • It hydrogen bonds to itself and to other surfaces causing CAPILLARY ACTION. • It holds large molecules together.

• Resists temperature change, helping to maintain a steady environment. • Expands when it freezes. Ice floats, cracks rocks (soil making).

Diffusion • All objects in motion have KINETIC ENERGY. • Robert Brown, Scottish science dude, 1827. Observes Brownian motion.
• Random motion of molecules. • Because of this random motion liquids and gases have a tendency to move from areas of high concentration to areas of low concentration.
• The net movement of particles from high conc. to low conc. • 3 key factors that effect the rate of diffusion.

• Temperature • Pressure • Concentration

Assignment • SA 6. 2 page 160 #1 -6.

LIFE SUBSTANCES

CARBON • Very Versatile. • Has 4 electrons available for bonding in its outer energy level.

CARBON • Can form bonds with other carbon atoms and other elements. • Carbon can form single, double, and triple bonds.
CARBON • Can form long chains. Straight, Branched and ringed. • Compounds with the same simple formula often have a different structure.
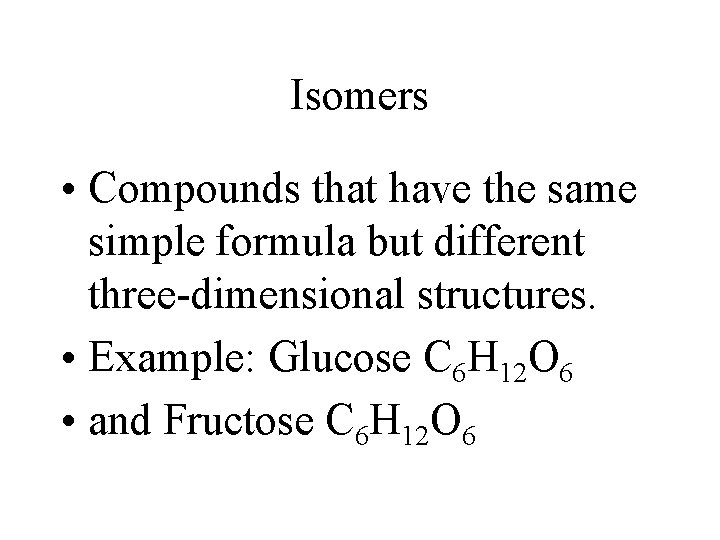
Isomers • Compounds that have the same simple formula but different three-dimensional structures. • Example: Glucose C 6 H 12 O 6 • and Fructose C 6 H 12 O 6
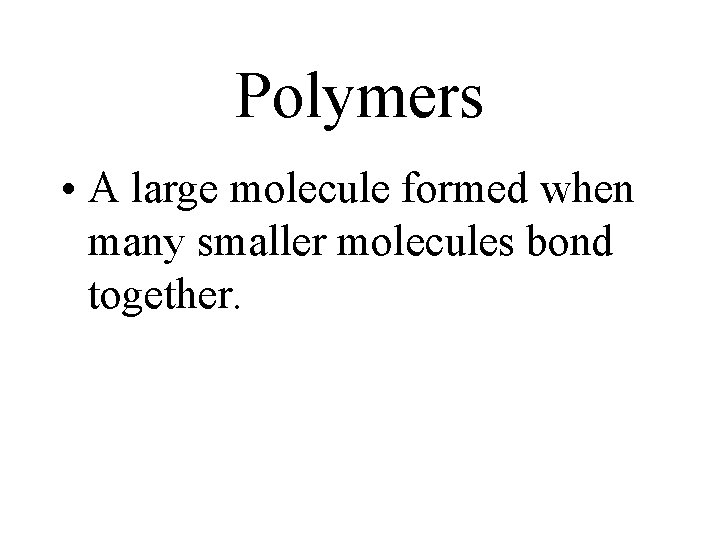
Polymers • A large molecule formed when many smaller molecules bond together.
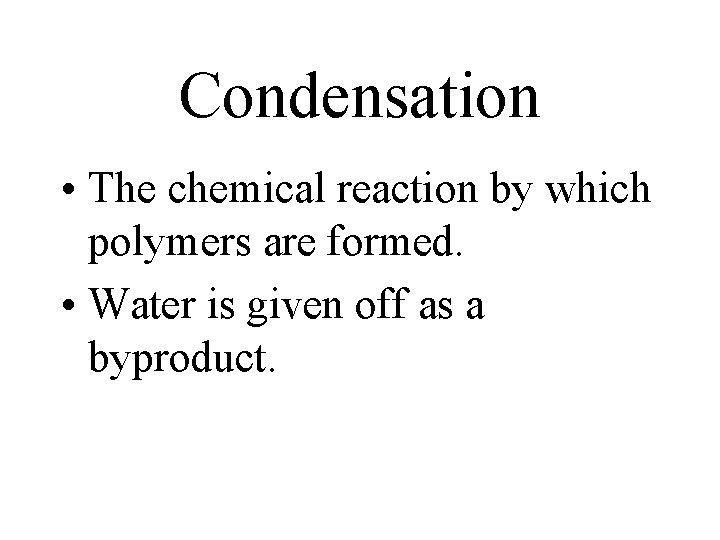
Condensation • The chemical reaction by which polymers are formed. • Water is given off as a byproduct.

Hydrolysis • A method by which polymers break apart. • Uses water to add H+ and OHions to the subunits that make up the polymer.

Carbohydrates • Organic compound composed of carbon, hydrogen and oxygen. • Ratio of 2 hydrogen atoms to every 1 oxygen atom for every carbon atom.

Carbohydrates • Used by the cell to store and release energy.
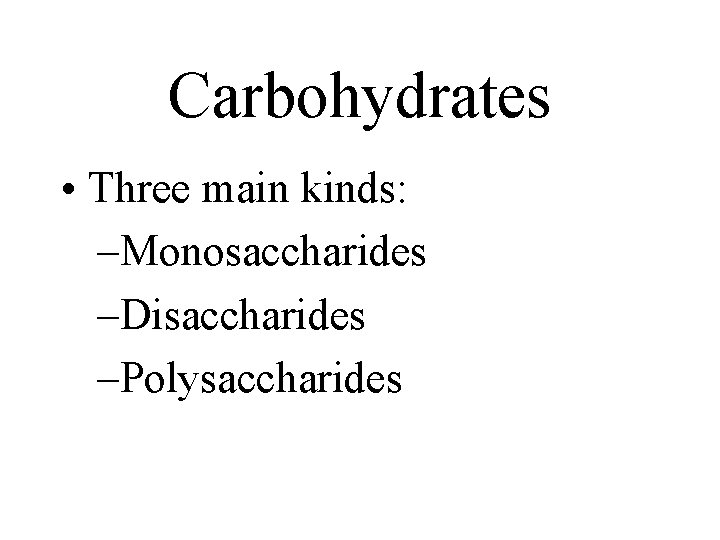
Carbohydrates • Three main kinds: –Monosaccharides –Disaccharides –Polysaccharides

Monosaccharides • Monosaccharide: single simple sugar. –Examples: Glucose, Fructose.

Disaccharides • When 2 Monosaccharides are linked together by a condensation reaction. –Example: Sucrose
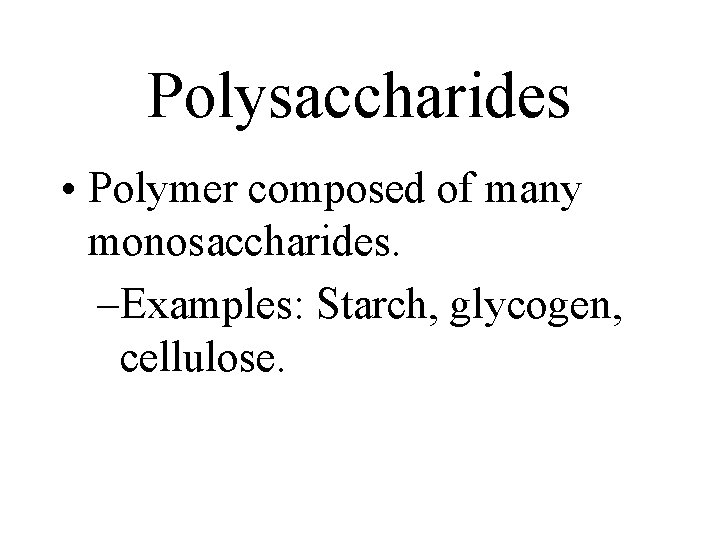
Polysaccharides • Polymer composed of many monosaccharides. –Examples: Starch, glycogen, cellulose.

Starch • Consists of highly branched chains of glucose units. • Used for food store in plants.

Glycogen • Very Highly branched • Used a food storage in Mammals. (stored in the liver)

Cellulose • Made of long chains of glucose units hooked together. • Used as structural support of plants.
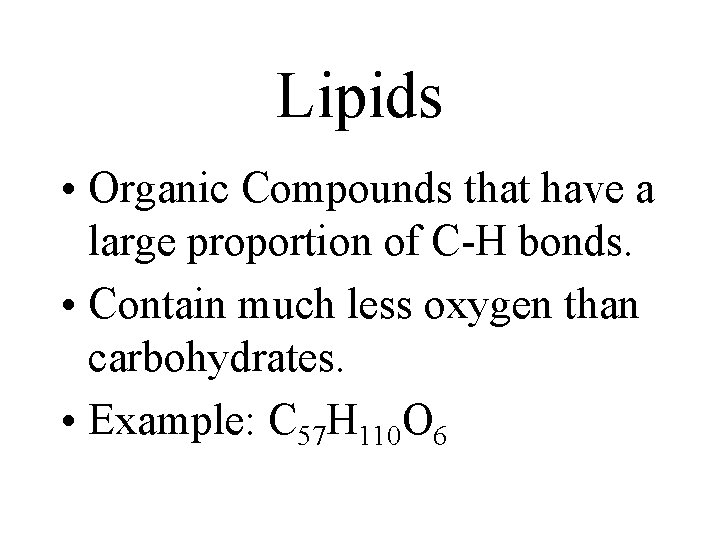
Lipids • Organic Compounds that have a large proportion of C-H bonds. • Contain much less oxygen than carbohydrates. • Example: C 57 H 110 O 6

Lipids • Commonly called FATS and OILS. • Insoluble in water. (nonpolar) • Cells use Lipids for energy storage, insulation, and protective coatings.

Lipids • Lipids are the major components of cell membranes that surround all living cells.
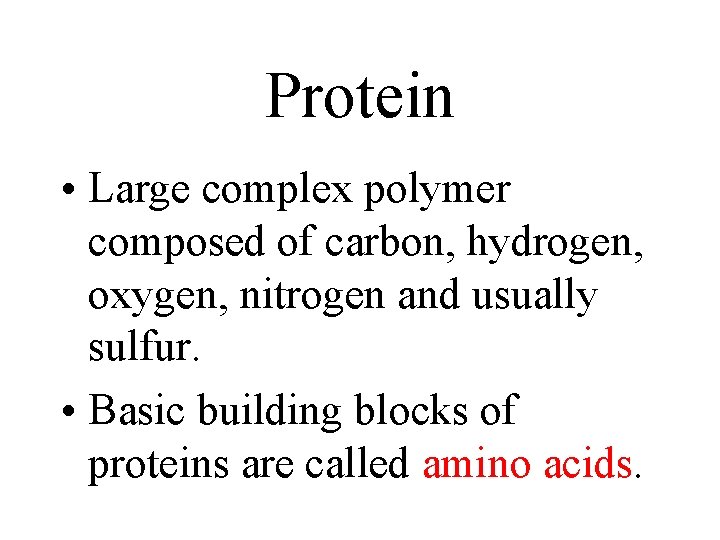
Protein • Large complex polymer composed of carbon, hydrogen, oxygen, nitrogen and usually sulfur. • Basic building blocks of proteins are called amino acids.

Protein • There are 20 different amino acids. • As A. A. are linked together proteins are formed.
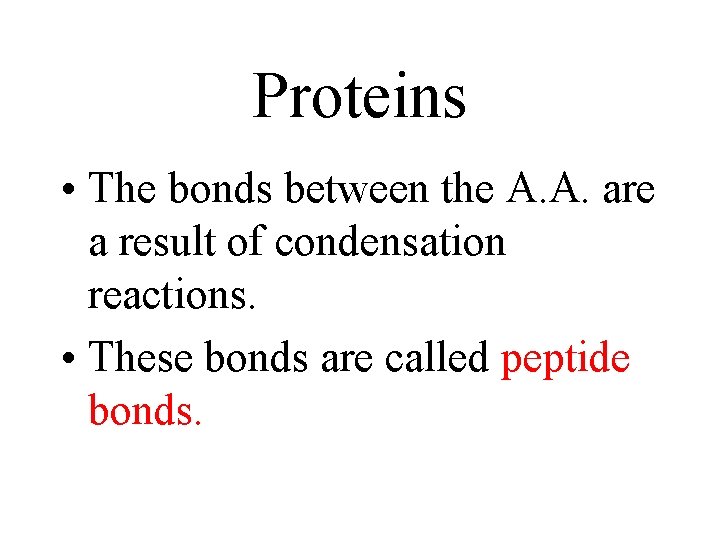
Proteins • The bonds between the A. A. are a result of condensation reactions. • These bonds are called peptide bonds.
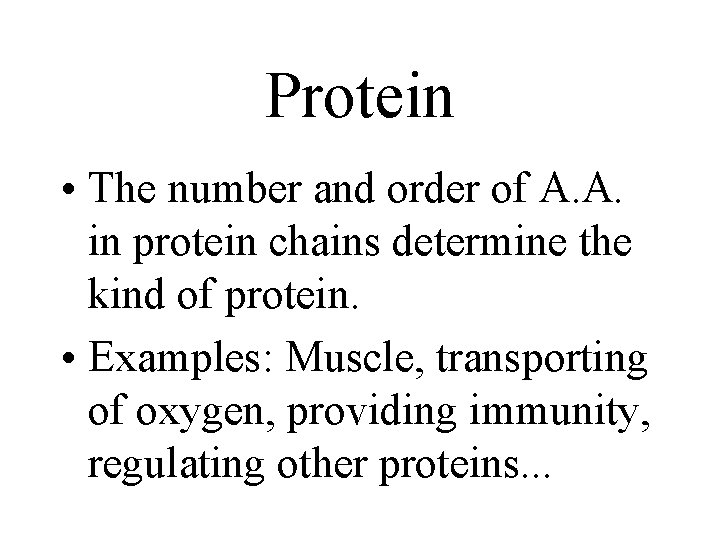
Protein • The number and order of A. A. in protein chains determine the kind of protein. • Examples: Muscle, transporting of oxygen, providing immunity, regulating other proteins. . .
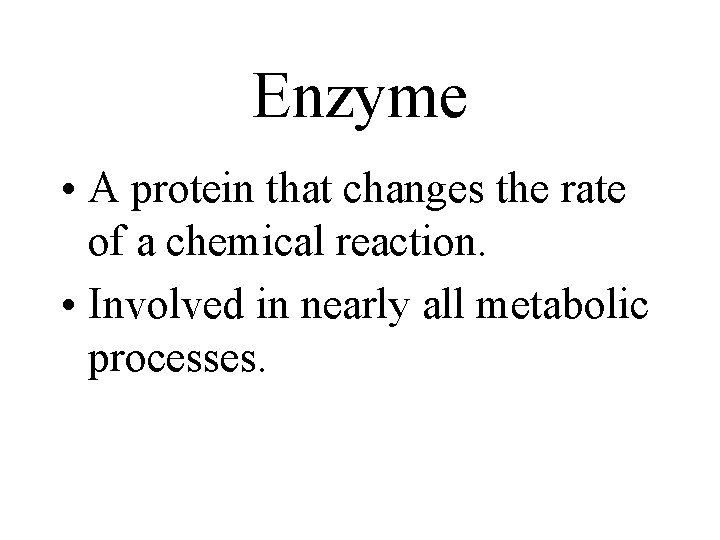
Enzyme • A protein that changes the rate of a chemical reaction. • Involved in nearly all metabolic processes.
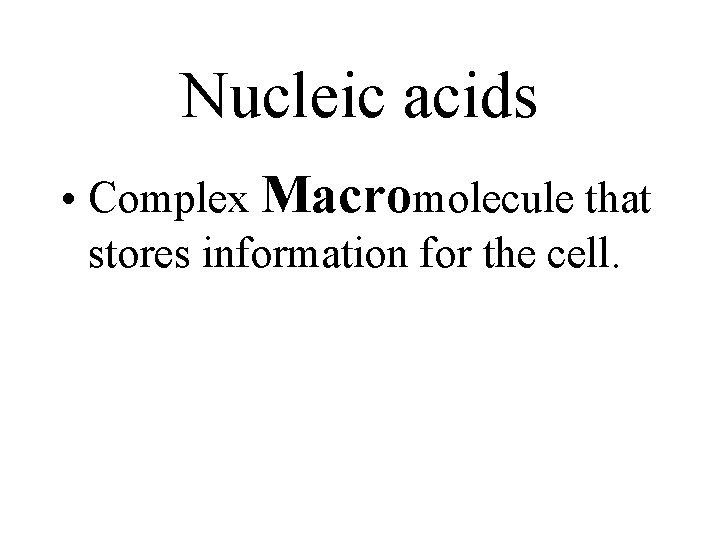
Nucleic acids • Complex Macromolecule that stores information for the cell.

• It’s made out of: –Carbon –Hydrogen –Oxygen –Nitrogen –Phosphorus

3 Main Parts • Nitrogen Base • Simple Sugar • Phosphate Group

2 Main Kinds • DNA • RNA

What’s it for? • RNA makes copies of DNA to make proteins.

Assignment • SA 6. 3 page 167, #1 -5. • CA 6 page 171 #1 -26.
- Slides: 58