The Chemistry of Life Atoms and their interactions














- Slides: 66

The Chemistry of Life

Atoms and their interactions z Element- a substance that can’t be broken down into simpler chemical substances y Everything is made of elements (Rocks, animals, plants, fungus) y On Earth, there are 90 naturally occurring elements

Atoms and their interactions z 25 of 90 naturally occurring elements are essential to living things z 4 of these 20 (Oxygen, Hydrogen, Nitrogen and Carbon) make up > 90% of the mass of a Human

Periodic Table of Elements z Symbol- Each elements is identified by a one or two-letter abbreviation z http: //www. periodictable. com/

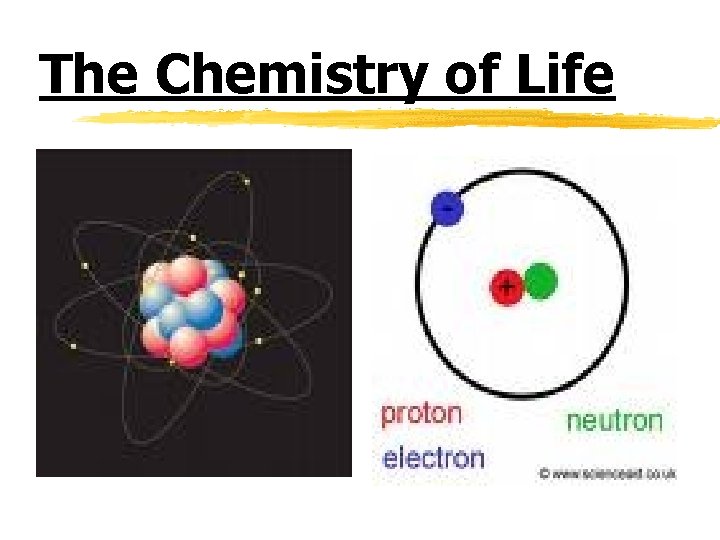
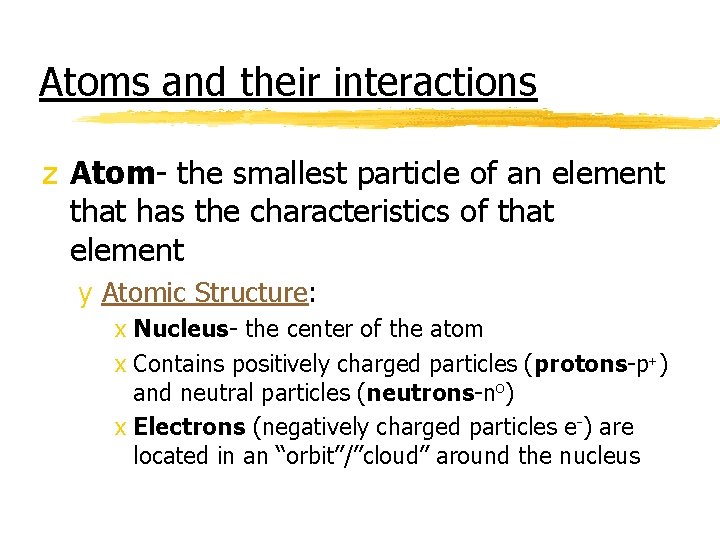
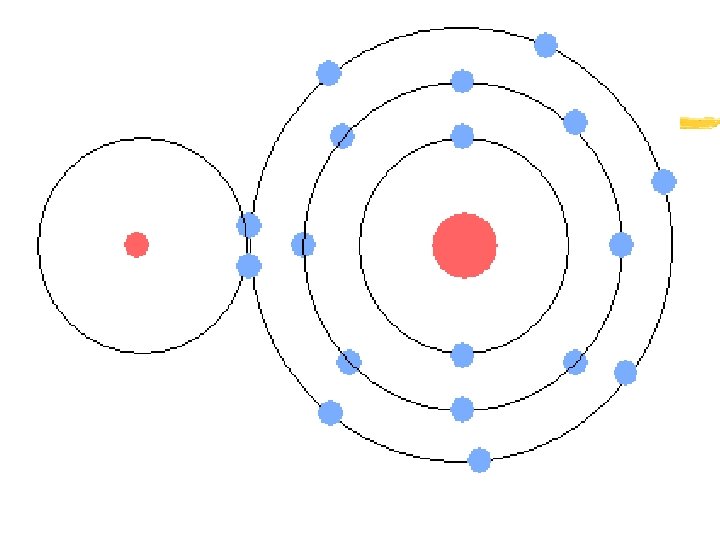
Atoms and their interactions z Atom- the smallest particle of an element that has the characteristics of that element y Atomic Structure: x Nucleus- the center of the atom x Contains positively charged particles (protons-p+) and neutral particles (neutrons-no) x Electrons (negatively charged particles e-) are located in an “orbit”/”cloud” around the nucleus

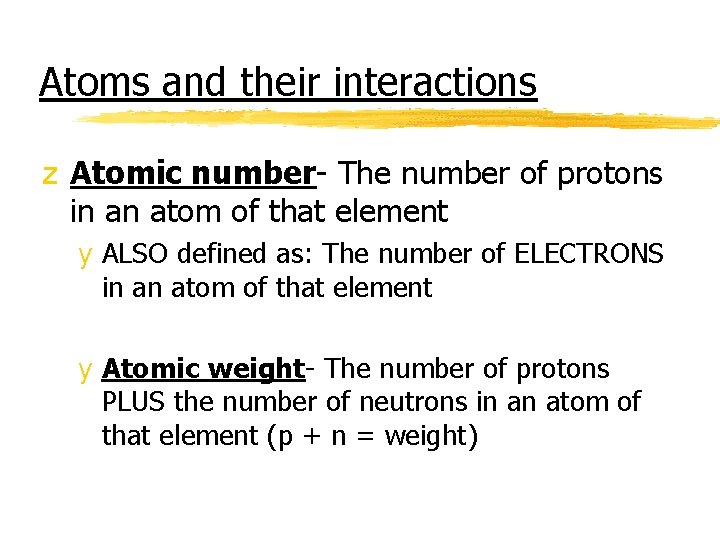
Atoms and their interactions z Atomic number- The number of protons in an atom of that element y ALSO defined as: The number of ELECTRONS in an atom of that element y Atomic weight- The number of protons PLUS the number of neutrons in an atom of that element (p + n = weight)

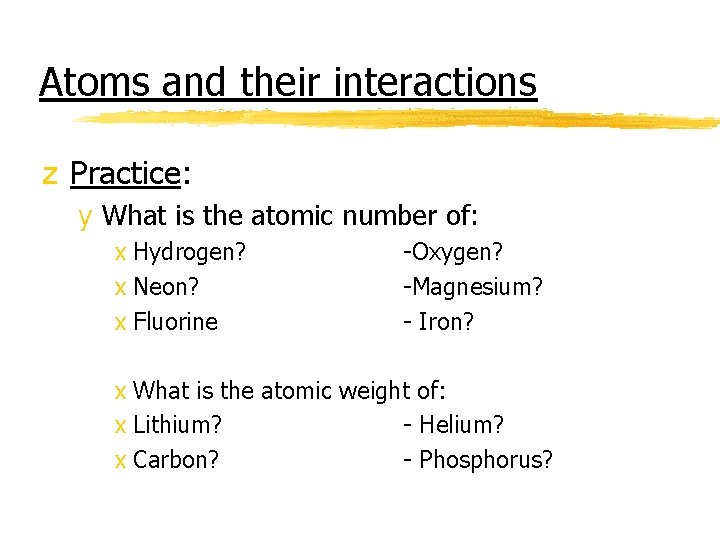
Atoms and their interactions z Practice: y What is the atomic number of: x Hydrogen? x Neon? x Fluorine -Oxygen? -Magnesium? - Iron? x What is the atomic weight of: x Lithium? - Helium? x Carbon? - Phosphorus?

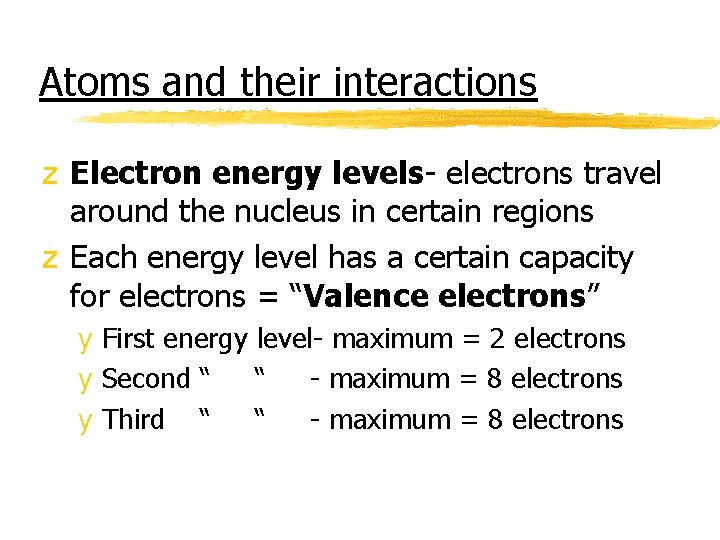
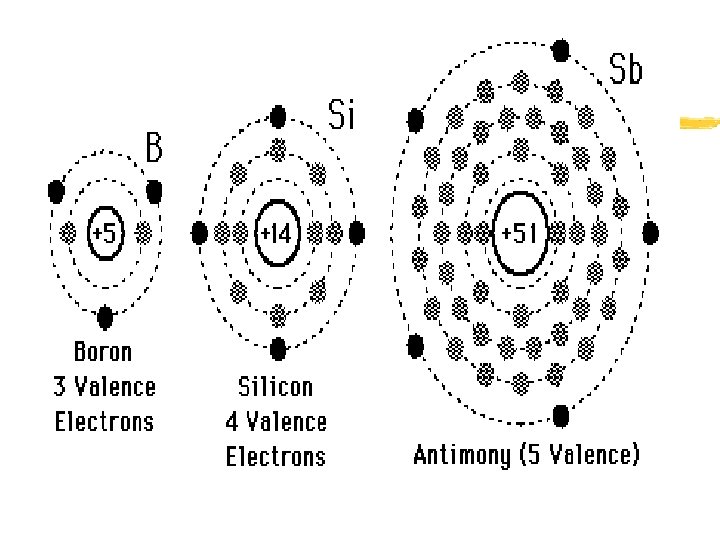
Atoms and their interactions z Electron energy levels- electrons travel around the nucleus in certain regions z Each energy level has a certain capacity for electrons = “Valence electrons” y First energy level- maximum = 2 electrons y Second “ “ - maximum = 8 electrons y Third “ “ - maximum = 8 electrons

Atoms and their interactions z Valence electrons- the total number of electrons in the HIGHEST energy level for that element y To determine number of valence electrons: x Determine elements’ atomic number x Begin to “load” electrons into energy levels, beginning with the first x After all electrons have been “loaded, ” determine the number of electrons element has in its OUTERMOST energy level


Atoms and their interactions z Practice: y What is the valence electron number for: x A. Hydrogen x B. Sulfer x C. Fluorine D. Magnesium E. Carbon F. Nitrogen

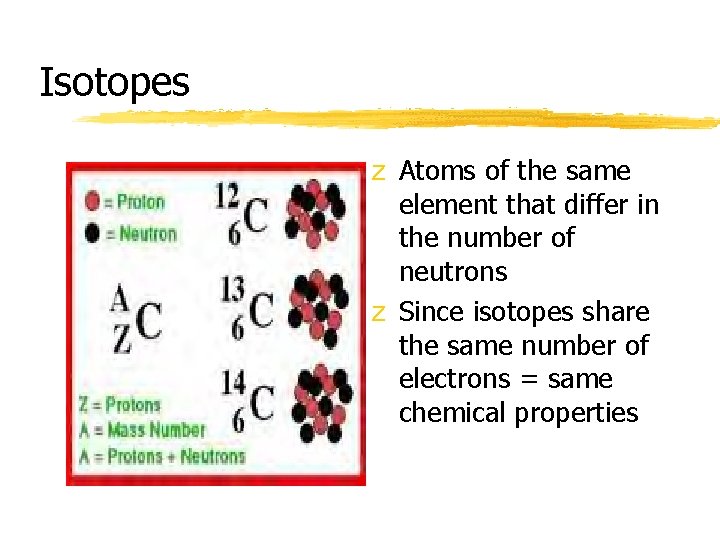
Isotopes z Atoms of the same element that differ in the number of neutrons z Since isotopes share the same number of electrons = same chemical properties

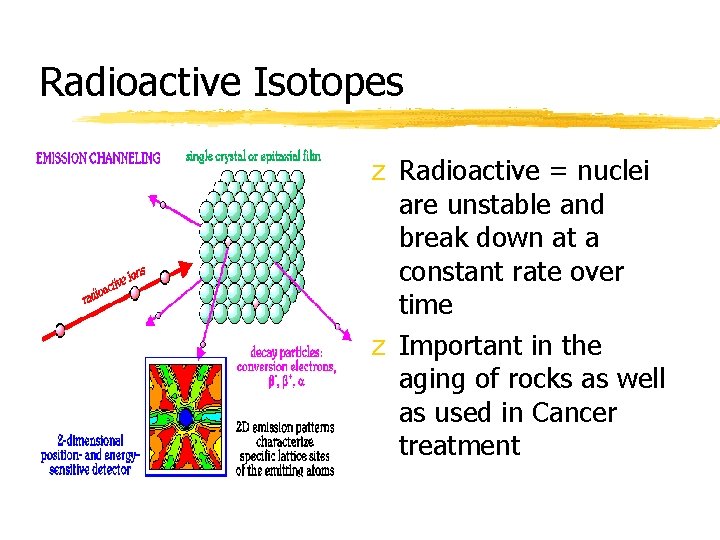
Radioactive Isotopes z Radioactive = nuclei are unstable and break down at a constant rate over time z Important in the aging of rocks as well as used in Cancer treatment


Compounds and Chemical Bonding z Compound- a substance that is composed of atoms of two or more different elements that are chemically combined z Examples: H 20, CH 4, and CO 2 z Chemical Bonding- Purpose is for elements to reach STABILITY z STABILITY = OUTERMOST ENERGY LEVEL of EACH ELEMENT are completely FILLED with MAXIMUM number of electrons

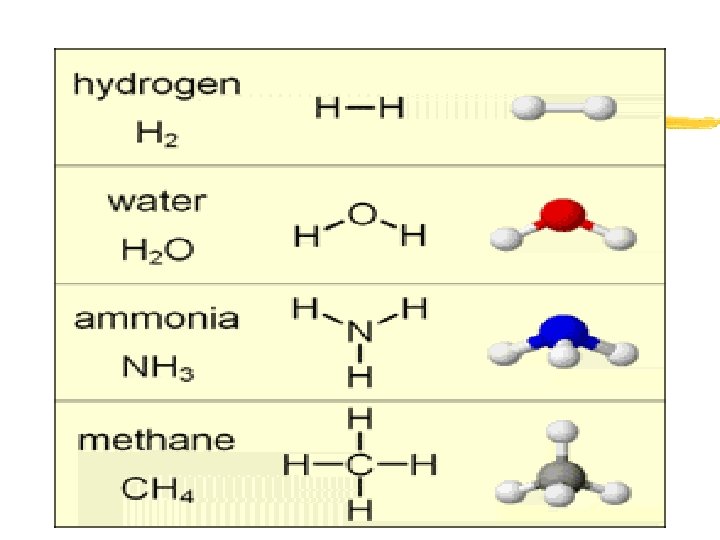
Covalent Bond z Bond that forms when valence electrons are shared between atoms z Covalent Bond- “sharing” of valence electrons z Examples: Hydrogen gas (H 2), Carbon Dioxide (CO 2), Water (H 2 O), Methane (CH 4) and Ammonia (NH 3) z Molecule- a group of atoms held together by covalent bonds and having no overall charge



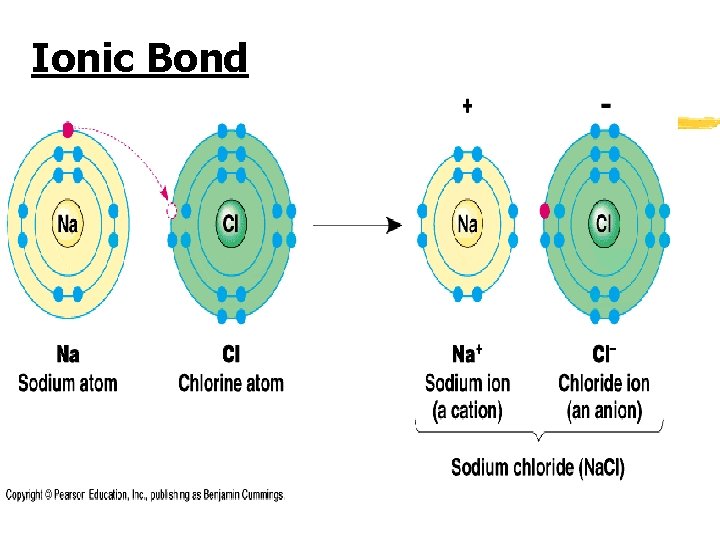
Ionic Bond z Formed when one or more electrons are transferred from one atom to another z Ionic Bond- “give-and-take” of valence electrons between elements z Examples: Sodium Chloride (Na. Cl), Beryillium Oxide (Be. O) and Magnesium Chloride (Mg. Cl 2)

Ionic Bond

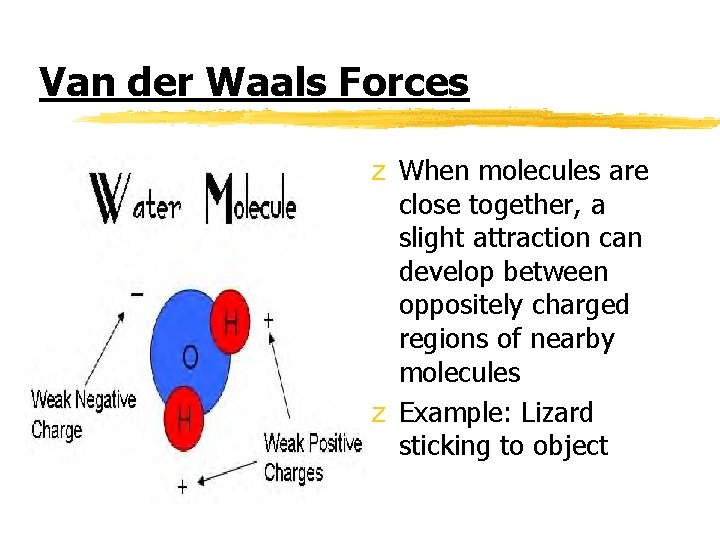
Van der Waals Forces z When molecules are close together, a slight attraction can develop between oppositely charged regions of nearby molecules z Example: Lizard sticking to object

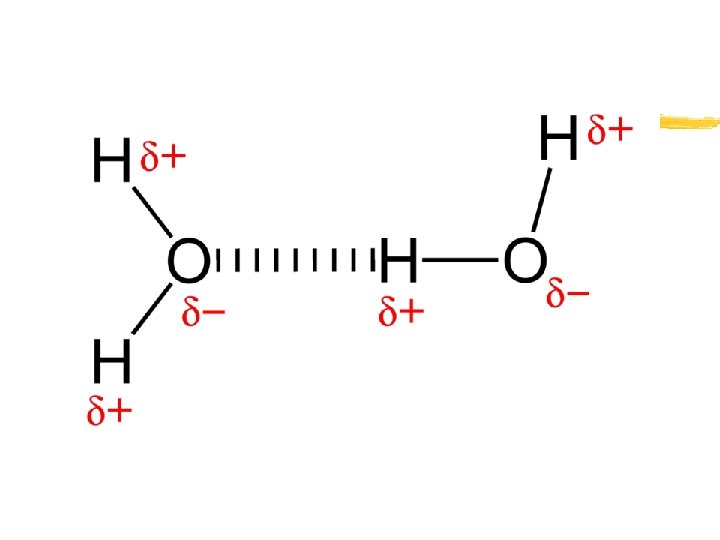
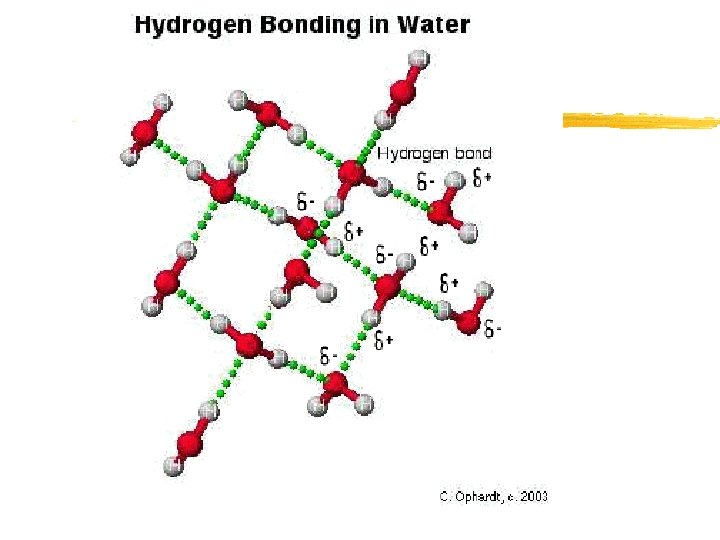
Properties of Water z Unique properties of water: y 1. Polarity- unequal distribution of electrons between the Oxygen and Hydrogen atoms within the molecule y 2. Hydrogen bonds- Due to the partial positive/negative charge on the Oxygen and Hydrogen atoms, water molecules are attracted to each other y Video of Hydrogen bonding



Properties of Water z Hydrogen bonds form between the partial negative charge on an oxygen atom and the partial positive charge on a neighboring hydrogen atom on a different water molecule z Cohesion-attraction between molecules of the same substance (water beads on a solid surface/rim of glass of water OR Insects walking on water) z Adhesion-attraction between molecules of different substances (Meniscus/fluid level-graduated cylinder) y Capillary action- water rising in a narrow tube against the force of gravity*NOTE>REDWOODS

Properties of Water z More properties of water: y Water EXPANDS as it freezes (Ice less dense than liquid water)-THANK GODZILLA! y Modulation of temperature: water absorbs heat from air that is warmer and releases stored heat to air that is cooler; Water effective as heat bank because it can absorb or release a tremendous amount of heat with only a slight change in its own temperature*Video

Water = Solvent of Life z Solution = a liquid that is a completely homogeneous mixture of two or more substances y Solvent- the dissolving agent of a solution y Solute- the substance being dissolved y Example: Dissolving salt (solute) in water (solvent); ultimately the glass will contain a uniform mixture of salt and water with salt concentration the same throughout the container y Video

Acids, Bases and p. H z Chemical reactions ONLY occur when conditions are PERFECT (Temperature, Pressure, availability of energy or a correct concentration of a particular substance dissolved in a solution) z p. H also plays a CRUCIAL role in whether or not a chemical reaction occurs

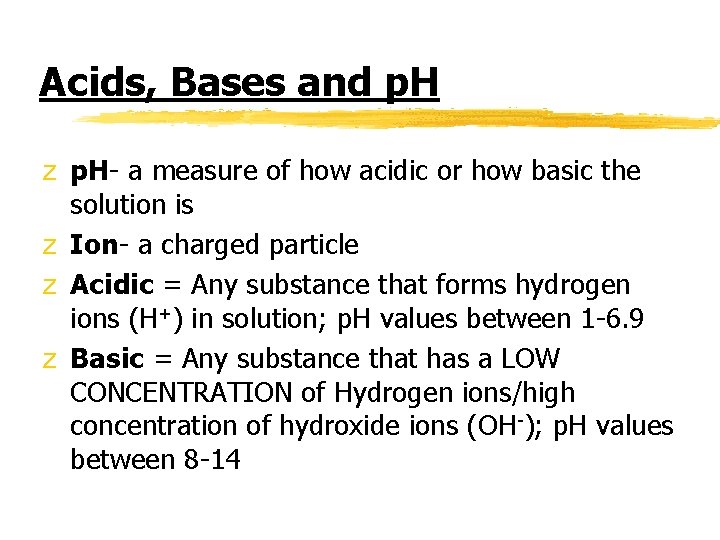
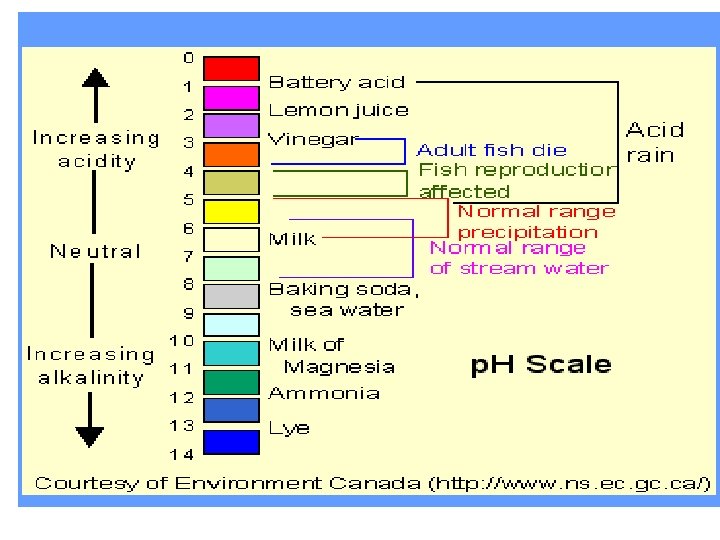
Acids, Bases and p. H z p. H- a measure of how acidic or how basic the solution is z Ion- a charged particle z Acidic = Any substance that forms hydrogen ions (H+) in solution; p. H values between 1 -6. 9 z Basic = Any substance that has a LOW CONCENTRATION of Hydrogen ions/high concentration of hydroxide ions (OH-); p. H values between 8 -14

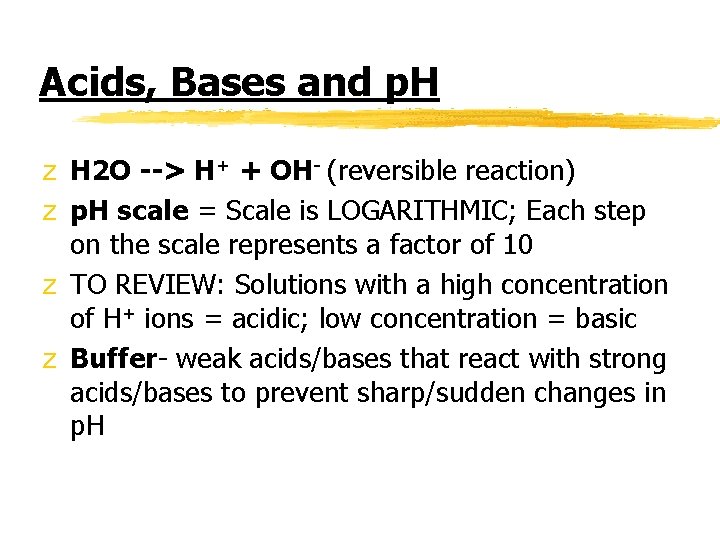
Acids, Bases and p. H z H 2 O --> H+ + OH- (reversible reaction) z p. H scale = Scale is LOGARITHMIC; Each step on the scale represents a factor of 10 z TO REVIEW: Solutions with a high concentration of H+ ions = acidic; low concentration = basic z Buffer- weak acids/bases that react with strong acids/bases to prevent sharp/sudden changes in p. H


Organic (Carbon) Chemistry z Organic Chemistry- The study of all compounds that contain bonds between Carbon atoms z Why is whole study of chemistry involved in studying Carbon compounds? y Carbon contains 4 valence electrons y Carbon can bond to other Carbon atoms

Organic (Carbon) Chemistry z Macromolecules- “giant molecules; ” Very large molecules made up of repeating subunits (monomers) of smaller molecules z Monomer- simplest/smallest subunit of macromolecule z Polymers- Many monomers joined together

Organic (Carbon) Chemistry z FOUR groups of organic compounds found in all living things: y Carbohydrates y Nucleic Acids - Lipids - Proteins y Polymerization- the process involved in linking monomers to form a polymer

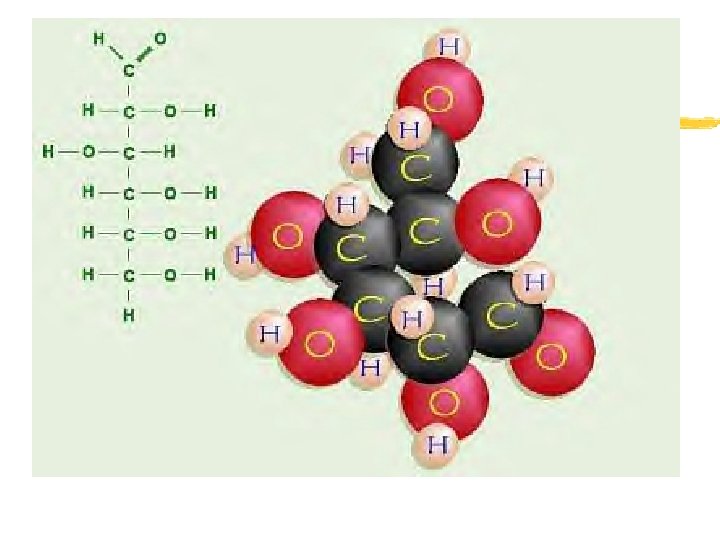
Carbohydrates z Carbohydrates- compounds made up of carbon, hydrogen and oxygen in a ratio of 1: 2: 1 z Function: Short term energy source (I. e. SUGAR) z In addition, some plants and animals use carbohydrates for structural purposes

Carbohydrates z Goal: breakdown of sugars (GLUCOSE) to supply ENERGY for all cell activities z Monosaccharides- simple sugars I. e. glucose (C 6 H 12 O 6) and fructose z Disaccharides- 2 monosaccharides linked together I. e. Sucrose (C 12 H 22 O 11) and Maltose



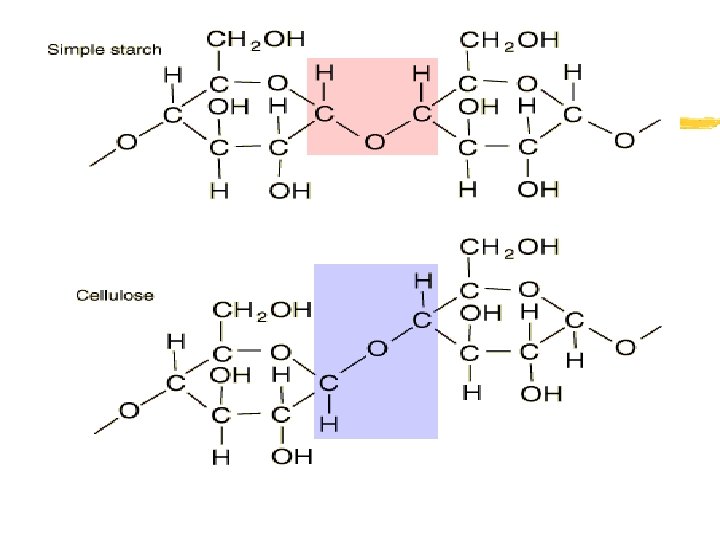
Carbohydrates z Polysaccharides- Many, many monosaccharides linked/bonded together. COMPLEX sugars! z Examples: Starch (excess energy storageplants), Cellulose (plant cell wall/structural factor) and Glycogen (excess sugar storage-animals)



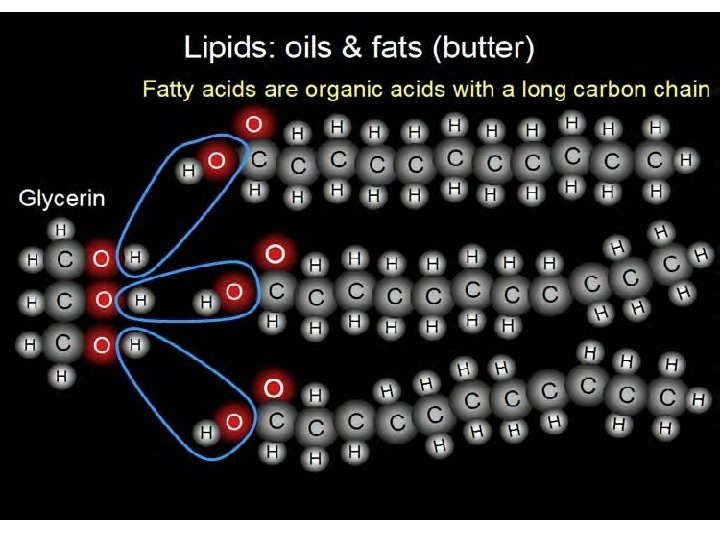
Lipids z Made up mostly of Carbon and Hydrogen atoms (greater than 2: 1 ratio of Hydrogen-Carbon bonds) along with less oxygen atoms-compared to Carbohydrates; NOT SOLUBLE in water! z Function: Long term energy storage z Function: Important parts of biological membranes that surround living cells z Function: Insulation

Lipids z Lipids are commonly referred to as Fats (Animal) and Oils (Plant) z Formed when a glycerol molecule combines with (3) fatty acid molecules z SATURATED = Each Carbon atom is joined to another Carbon by SINGLE bond z UNSATURATED= At least ONE Carbon-Carbon DOUBLE BOND


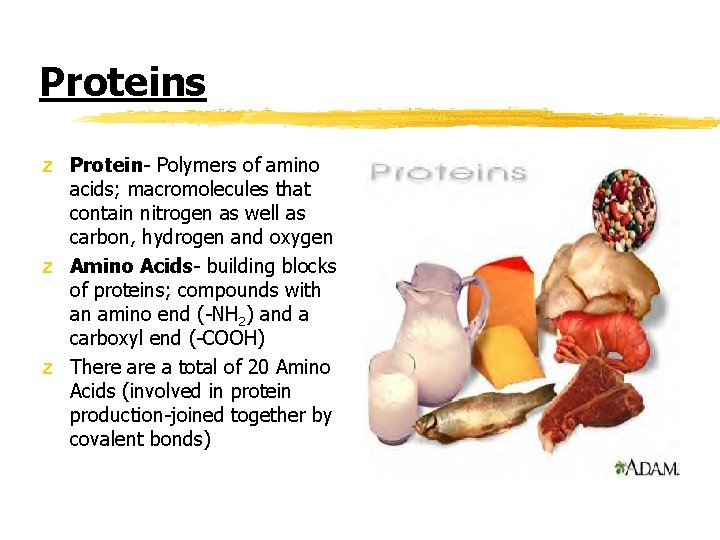
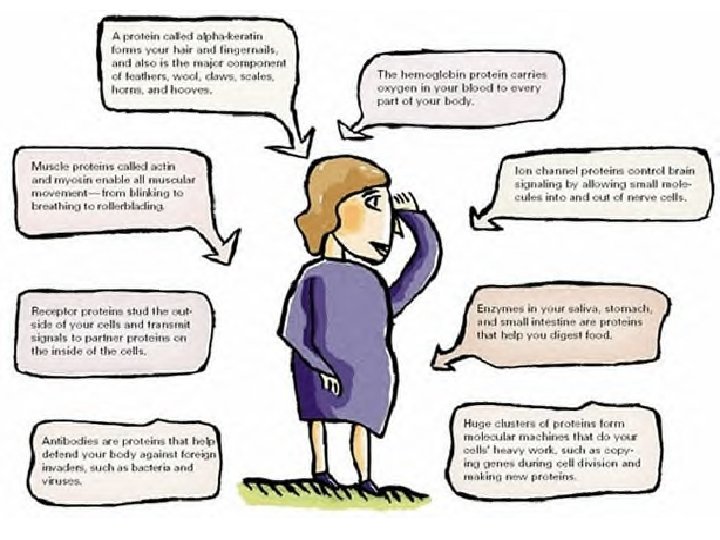
Proteins z Protein- Polymers of amino acids; macromolecules that contain nitrogen as well as carbon, hydrogen and oxygen z Amino Acids- building blocks of proteins; compounds with an amino end (-NH 2) and a carboxyl end (-COOH) z There a total of 20 Amino Acids (involved in protein production-joined together by covalent bonds)

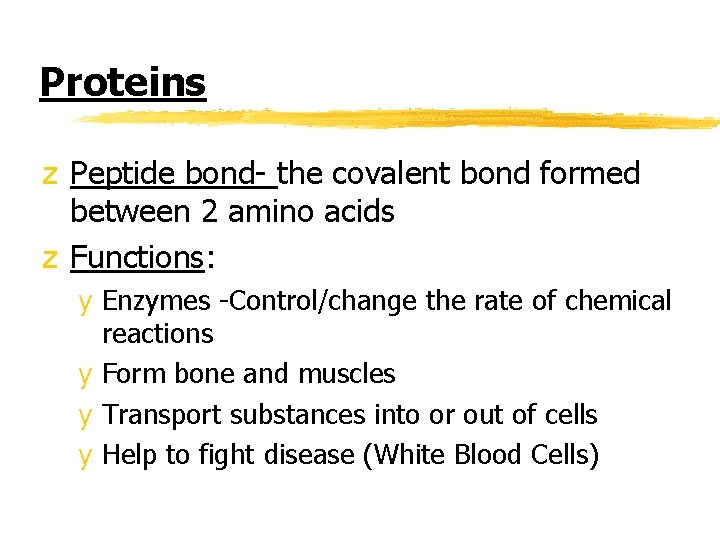
Proteins z Peptide bond- the covalent bond formed between 2 amino acids z Functions: y Enzymes -Control/change the rate of chemical reactions y Form bone and muscles y Transport substances into or out of cells y Help to fight disease (White Blood Cells)


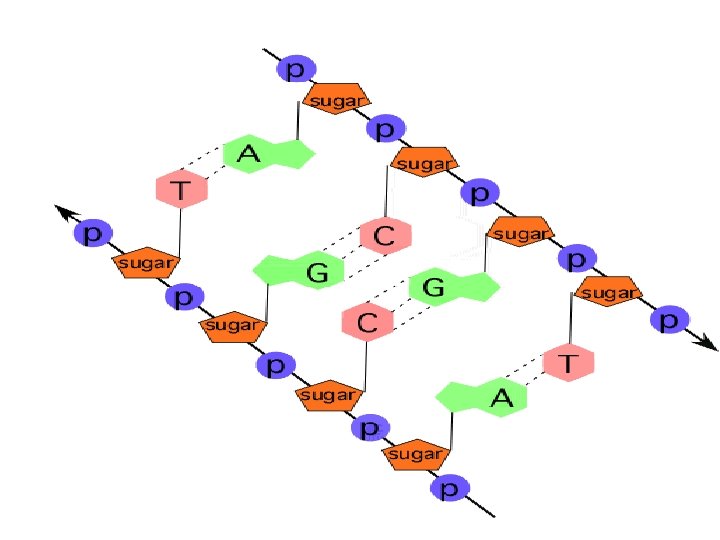
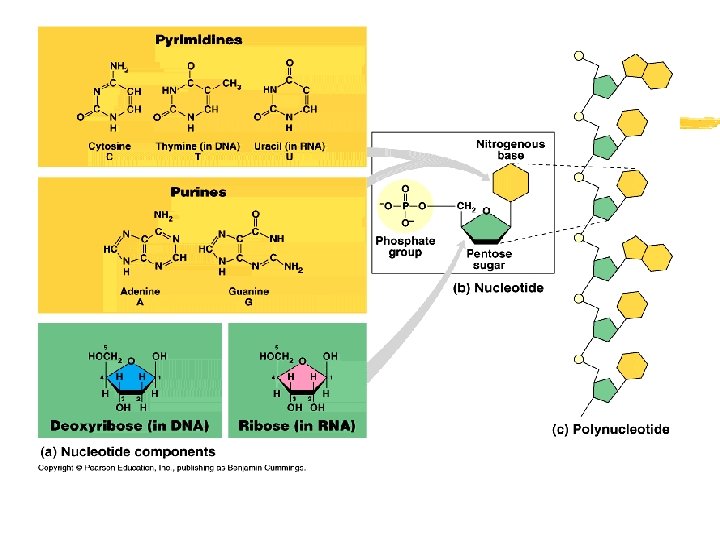
Nucleic Acids z A complex macromolecule that stores cellular information in the form of a code (the GENETIC CODE) z Macromolecules made up of carbon, hydrogen, oxygen, nitrogen and phosphorus z Nucleotide- 5 carbon sugar + Phosphate group + Nitrogenous base z Many nucleotides paired together z Examples: DNA and RNA

Nucleic Acids z DNA = master copy of organism’s information code z Information encoded used to form all of the organisms’ enzymes and structural proteins; determines what organism looks like and how it acts z RNA- forms a copy of DNA for use in making proteins



Chemical Reactions z Chemical reaction- the breaking and reforming of chemical bonds, with the subsequent “exchange” of atoms z Metabolism- All of the chemical reactions that occur within an organism; an organism’s metabolism

Chemical Reactions z As mentioned, chemical reactions ALWAYS involve the breaking of bonds in reactants and the formation of new bonds in products z Energy is released or absorbed whenever chemical bonds form or are broken

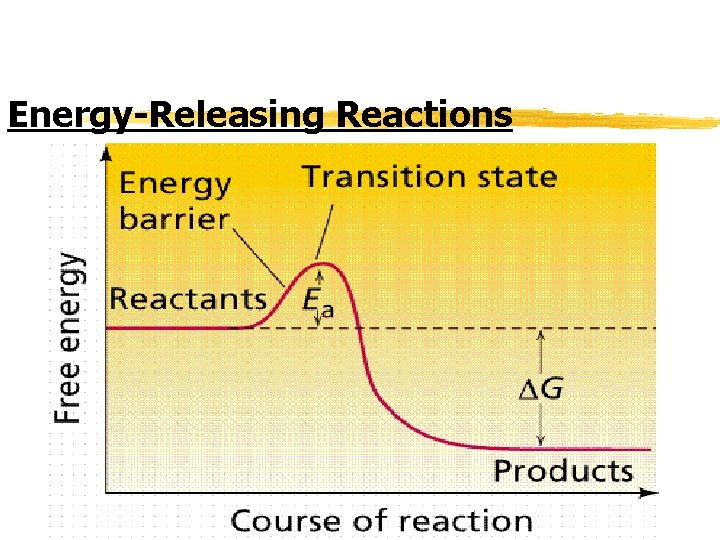
Chemical Reactions z Energy changes: y Chemical reactions that release energy often occur SPONTANEOUSLY y Chemical reactions that absorb energy WILL NOT occur without a source of energy y Example of Energy-releasing reaction is hydrogen gas burning/reacting with oxygen to produce water vapor (gas)

Chemical Reactions z 2 H 2 + O 2 --> 2 H 2 O y The energy is released (form of heat) or sometimes-when hydrogen gas explodes-light and sound released y Reverse reaction (water converted into hydrogen and oxygen gas) absorbs SO MUCH ENERGY that doesn’t occur by itself y Therefore, one direction energy released and other direction-energy required

Energy-Releasing Reactions

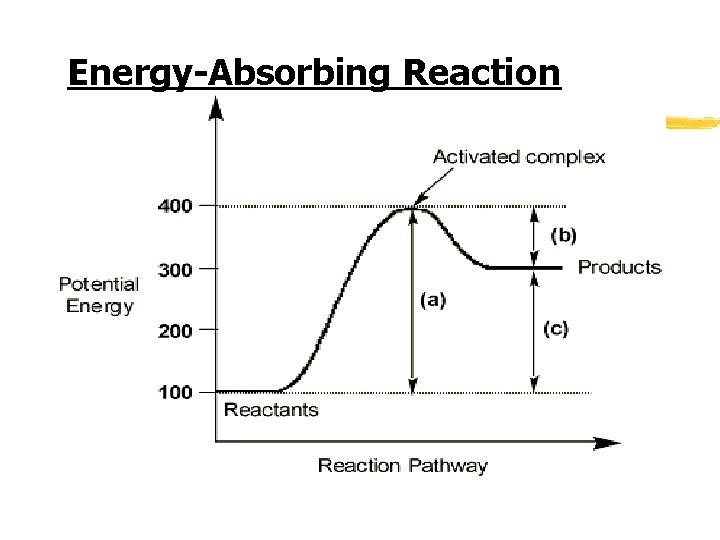
Energy-Absorbing Reaction

Energy Changes z Q: What significance do these energy changes have on living things? y To stay alive, organisms need to carry out reactions that require energy y Every organism must have a source of energy to carry out chemical reactions y Plants get energy-trap and store energy in energyrich compounds y Animals get energy by consuming plants/animals

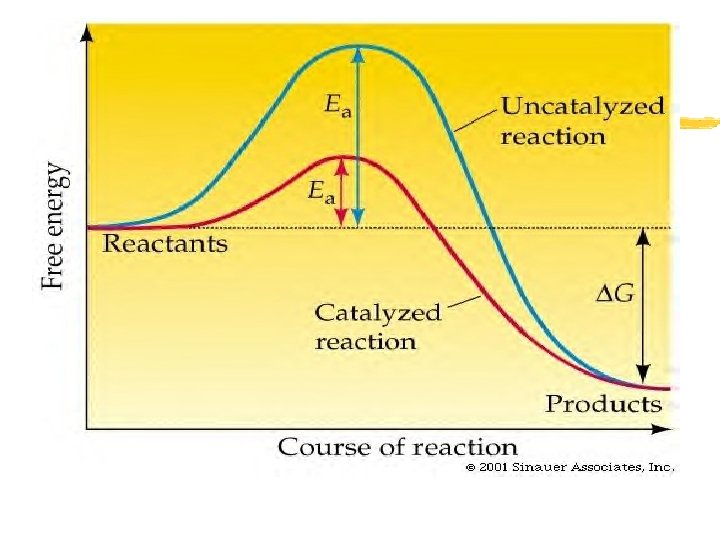
Activation Energy z Activation energy- the energy required to start a chemical reaction z Factor in whether the overall chemical reaction releases energy or absorbs energy

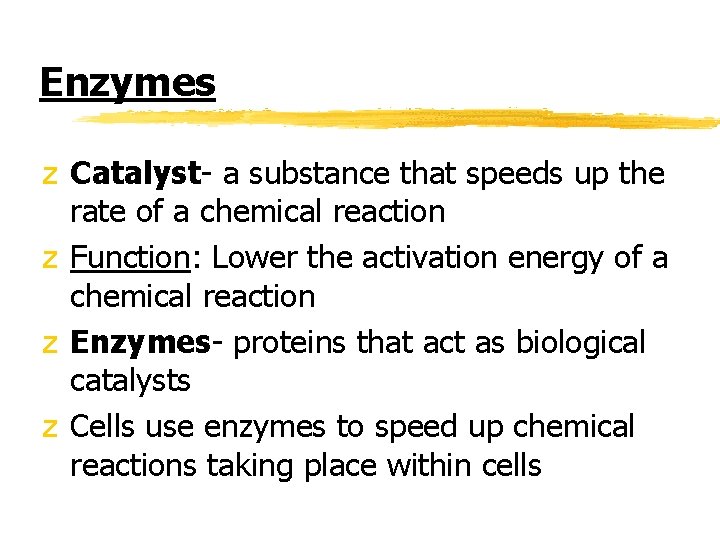
Enzymes z Catalyst- a substance that speeds up the rate of a chemical reaction z Function: Lower the activation energy of a chemical reaction z Enzymes- proteins that act as biological catalysts z Cells use enzymes to speed up chemical reactions taking place within cells


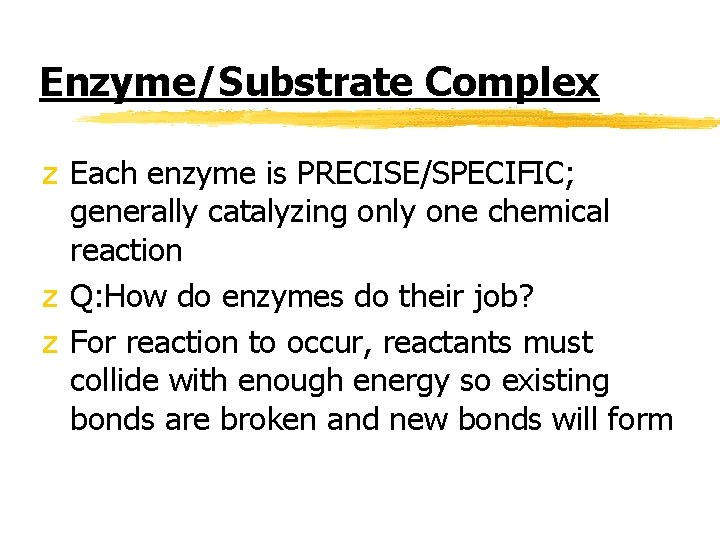
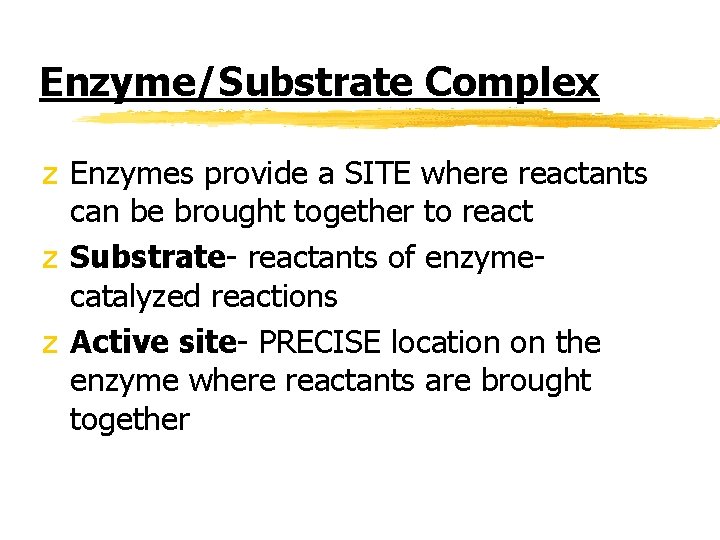
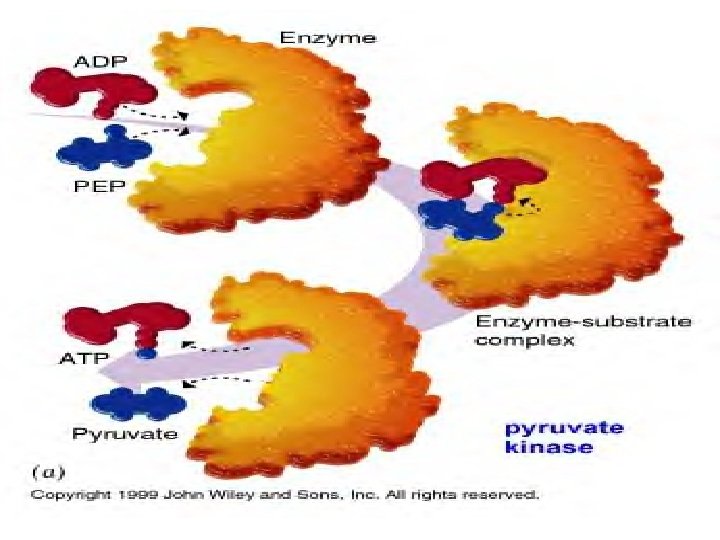
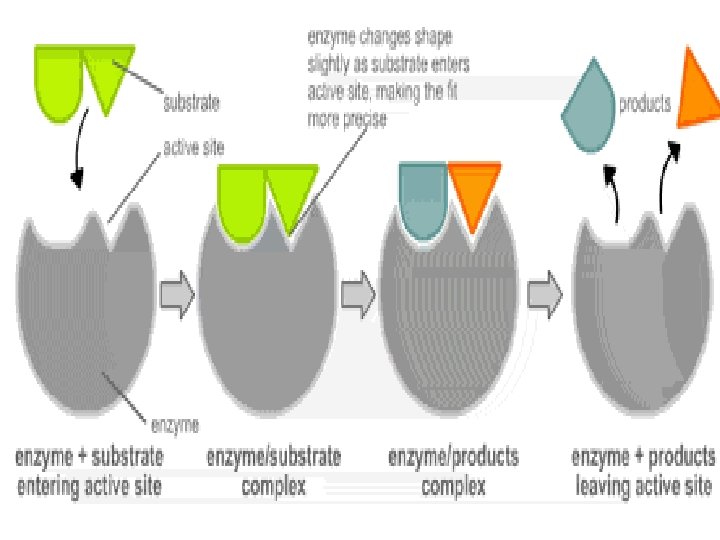
Enzyme/Substrate Complex z Each enzyme is PRECISE/SPECIFIC; generally catalyzing only one chemical reaction z Q: How do enzymes do their job? z For reaction to occur, reactants must collide with enough energy so existing bonds are broken and new bonds will form

Enzyme/Substrate Complex z Enzymes provide a SITE where reactants can be brought together to react z Substrate- reactants of enzymecatalyzed reactions z Active site- PRECISE location on the enzyme where reactants are brought together



Regulation of Enzyme Activity z Factors that affect enzyme activity: y p. H y Changes in Temperature y Cell action; contain proteins that turn key enzymes “on” or “off” at critical stages in the life of a cell