The Chemical Level of Organization DO NOW Sept












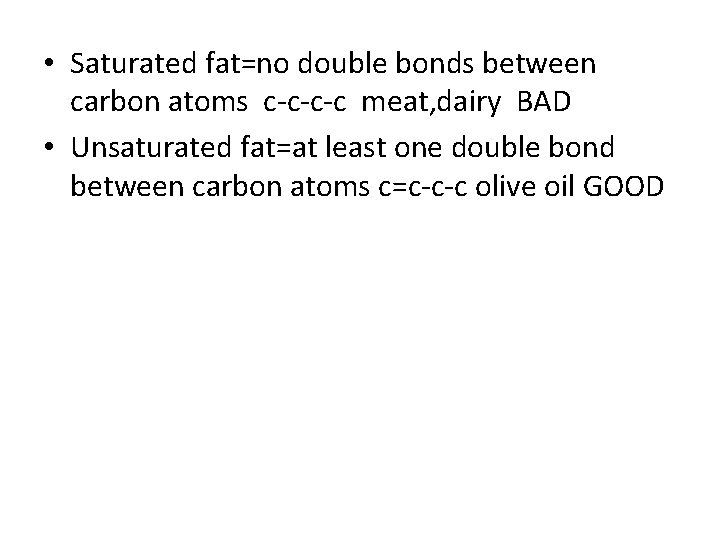


- Slides: 44

The Chemical Level of Organization

DO NOW Sept 29 • Why is water called a polar molecule? • Why is this property important to the function of living things?

DO NOW ANSWER • Oxygen and Hydrogen atoms carry slight charges. • Dehydration and Hydrolysis reactions contribute to the body’s overall metabolism. • Assists in maintaining homeostasis. • Serves as a medium for the cell to perform many of its processes.

AGENDA Sept 29 • Objective: Review the molecular level of organization and structure in living things. • 1. Biology and Chemistry Review • 2. Review and Homework – Use the notes on my teacher webpage to review. – Articles DUE tomorrow.

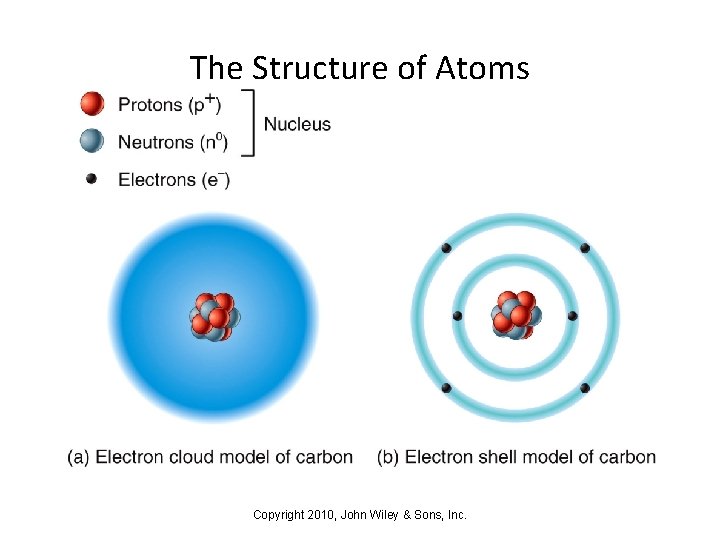
Chapter 2: The Chemical Level of Organization • Understanding how our bodies function requires that we understand appreciate the chemical level of organization • All matter - including your body - is made up of atoms • Matter can exist in 3 forms: – Solid [e. g. ice is the solid phase of water] – Liquid [water as a liquid has a definite volume, but no set shape] – Gas [water vapor is a gas - both volume and shape can change] • Each chemical element is made of a different type of atom • Four elements (oxygen, carbon, hydrogen, and nitrogen) account for over 96% of our body mass Copyright 2010, John Wiley & Sons, Inc.

The Structure of Atoms Copyright 2010, John Wiley & Sons, Inc.

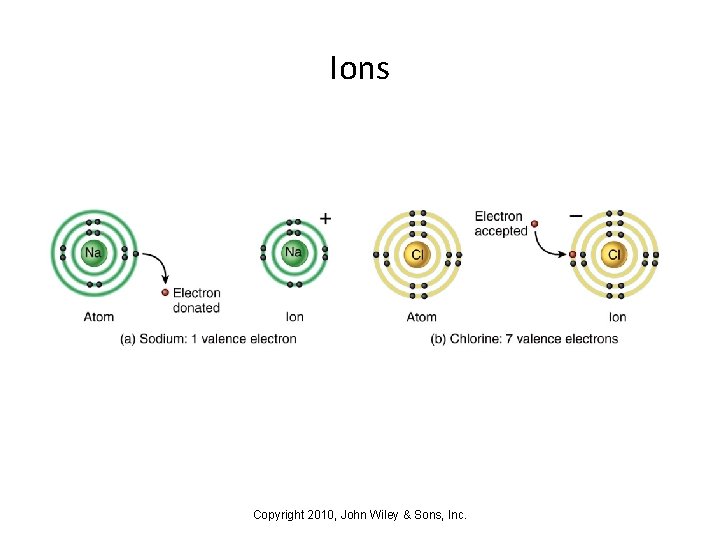
Ions • Ions are atoms that have gained or lost an electron. Ions have a net electrical charge. • When atoms lose an electron, they are left with a positive charge and are called cations (e. g. , Na+, K+, Ca 2+). • When atoms gain an electron, they are left with a negative charge and are called anions (e. g. , Cl–, Br–). Copyright 2010, John Wiley & Sons, Inc.

Ions Copyright 2010, John Wiley & Sons, Inc.

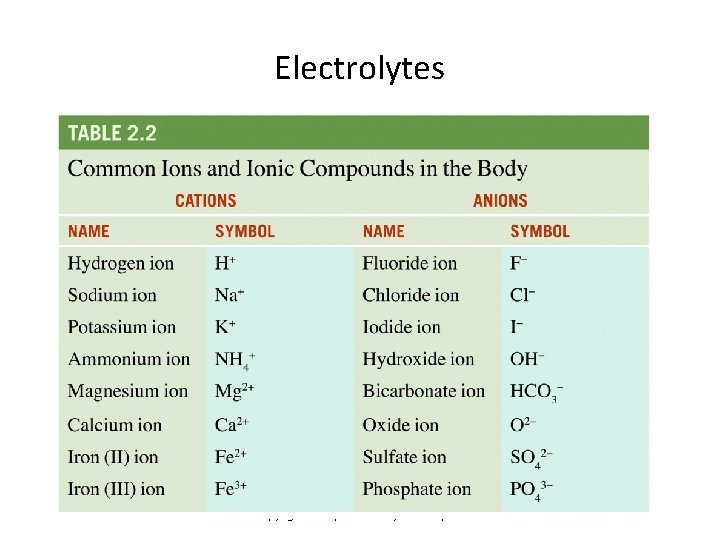
Electrolytes • In water, ionic compounds dissociate into separate ions • These separate dissolved ions are called electrolytes • Most ions in the body are dissolved in various body fluids as electrolytes Copyright 2010, John Wiley & Sons, Inc.

Electrolytes Copyright 2010, John Wiley & Sons, Inc.

Inorganic Compounds • • Lack carbon Structurally simple Held together by ionic or covalent bonds Water, salts, acids, bases, carbon dioxide, bicarbonate ion

Water • • • Makes up 55 -60% of body mass All chem reactions occur in a watery medium Polar Solvent Absorb or release heat Lubricant (saliva, mucus)

Ph • • • Measures acidity and alkalinity of a solution Acid contains more H+ than pure water Bases contain more OH- than pure water Must remain constant for body to be healthy Blood p. H =7 Ph is maintained by a buffer system. A buffer is a weak acid or base. Eliminates H+ or OH- in order to maintain p. H homeostasis

Organic Compounds • • • Contain carbon Carry out complex functions Held together by covalent bonds Larger than inorganic Carbohydrates, proteins, lipids, nucleic acid

Carbohydrates • • Sugars and starches Provide energy Single sugar=monosaccharide Glucose, galactose, fructose Double sugar=disaccharide Lactose sucrose Complex sugar=polysaccharide Starch, glycogen(released from liver to increase glucose)


AGENDA Sept 30 • Objective: Review the molecular level of organization and structure in living things. • 1. Biology and Chemistry Review – Four Classes of Organic Compounds • 2. Review and Homework – Use the notes on my teacher webpage to review. – Hand in Articles. • Organize them per topic and Staple them!

Carbohydrates - Building Blocks and Energy Sources • Carbohydrates play a key role in cells as a source of chemical energy for producing ATP • Carbohydrates contain C, H, and O atoms • Carbohydrates usually have names that end in –ose e. g. glucose, galactose, maltose • The three main groups of carbohydrates are: – Monosaccharides – Disaccharides – Polysaccharides • Carbohydrates play important structural roles in some molecules (notably ribose and deoxyribose in nucleic acids). Copyright 2010, John Wiley & Sons, Inc.

Simple Sugars - Monosaccharides and Disaccharides • Monosaccharides are single sugar molecules – Glucose and fructose are two common examples • Disaccharides form when two single sugars combine – Lactose and sucrose are both disaccharides found in our food Copyright 2010, John Wiley & Sons, Inc.

Polysaccharides - Polymers of Monosaccharides • Polysaccharides result from additional dehydration synthesis reactions – Glycogen is an important storage form of glucose in our bodies – Starch (amylose) is a major polysaccharide in our diet and the main storage form of glucose in plants. – Cellulose is an important structural molecule in plants, and is also known as dietary fiber when found in food Copyright 2010, John Wiley & Sons, Inc.

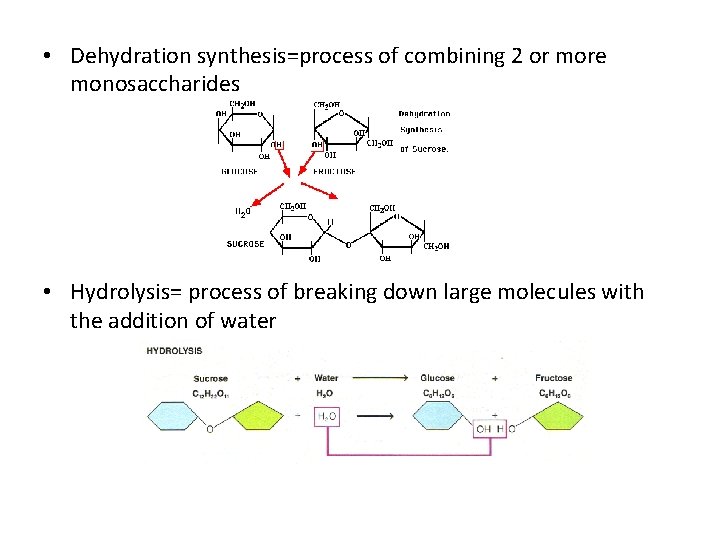
• Dehydration synthesis=process of combining 2 or more monosaccharides • Hydrolysis= process of breaking down large molecules with the addition of water

Lipids - A Diverse Class of Non-Polar Molecules • Lipids contain mostly carbon and hydrogen atoms, with only a few oxygen atoms • Their small number of polar covalent bonds results in lipid molecules being non-polar and hydrophobic • Major classes of lipids include: – Fatty acids – Triglycerides (fats and oils) – Phospholipids – Steroids Copyright 2010, John Wiley & Sons, Inc.

Fatty Acids - The Simplest Lipids • These molecules contain a non-polar chain of carbon atoms (the “fatty” portion of the molecule) bound to a carboxylic acid group • Unsaturated fatty acids contain C – C double bonds while saturated fatty acids do not (they are “saturated” with hydrogen atoms) • Monounsaturated and polyunsaturated fatty acids contain one or multiple double bonds respectively Copyright 2010, John Wiley & Sons, Inc.

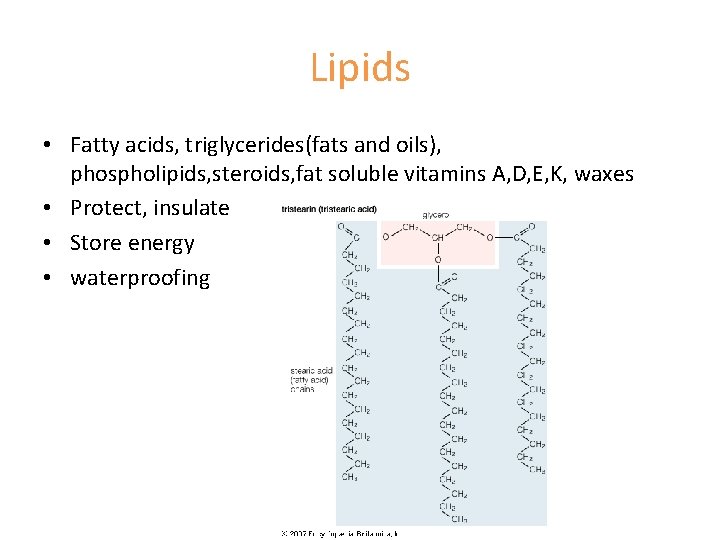
Lipids • Fatty acids, triglycerides(fats and oils), phospholipids, steroids, fat soluble vitamins A, D, E, K, waxes • Protect, insulate • Store energy • waterproofing
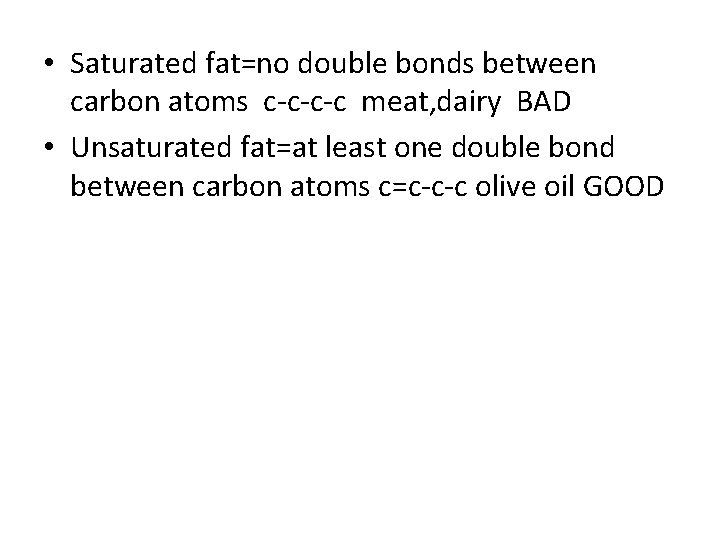
• Saturated fat=no double bonds between carbon atoms c-c-c-c meat, dairy BAD • Unsaturated fat=at least one double bond between carbon atoms c=c-c-c olive oil GOOD

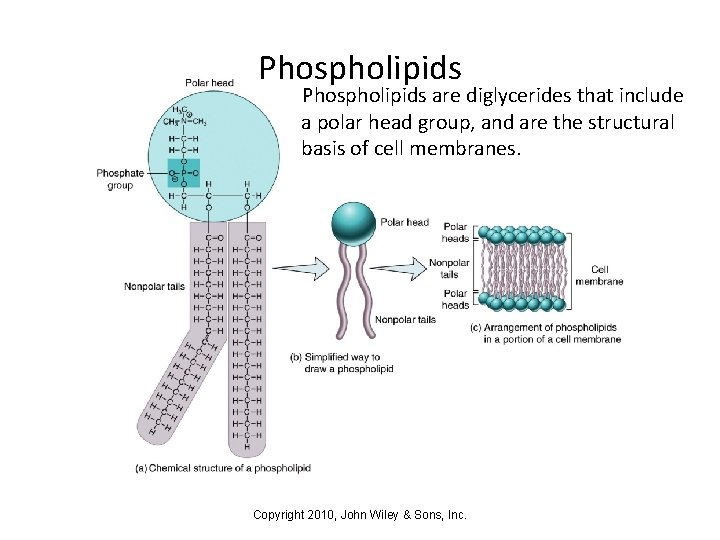
Phospholipids are diglycerides that include a polar head group, and are the structural basis of cell membranes. Copyright 2010, John Wiley & Sons, Inc.

Steroids • All steroids contain 4 linked carbon rings, and are made from cholesterol: – Cholesterol is an important component of cell membranes and a precursor for all other steroids – Steroid hormones - chemical signals regulating homeostasis – Bile acids - detergent-like component of bile important for lipid digestion – Anabolic steroids+ group of lipids that stimulate anabolic metabolism and promote growth of certain tissues • Used to treat certain disease conditions • increase muscle mass – Adverse changes in liver function – Increase risk of heart attack – Increase risk of blood vessel disease – Upsets hormonal balance---mood change, severe psychological disorders Copyright 2010, John Wiley & Sons, Inc.

Proteins - A Key Class of Molecules in Living Systems • Proteins are important molecules for building body structure and determining function, e. g. , collagen, hemoglobin, enzymes • Proteins vary a lot in terms of size and chemical make-up, with many proteins having very complex shapes Copyright 2010, John Wiley & Sons, Inc.

Proteins Collagen, human hair, keratin Composed of amino acids 20 different amino acids The “R” group determines the type of a. a. Provides protection, help muscles contract, transport substances in and out of cells • Enzymes= catalyst that speeds up chemical reactions (ase) • • •

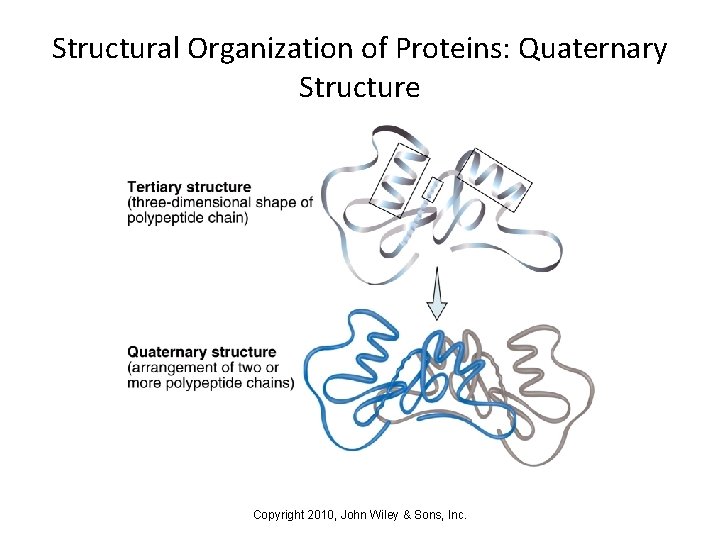
Structural Organization of Proteins - Summary • The 4 levels of protein structure: – Primary structure is the sequence of amino acids – Secondary structure involves local twisting or folding of the polypeptide backbone – Tertiary structure refers to the overall 3 -dimensional shape of a protein – Quaternary structure occurs when 2 or more polypeptides interact with one another Copyright 2010, John Wiley & Sons, Inc.

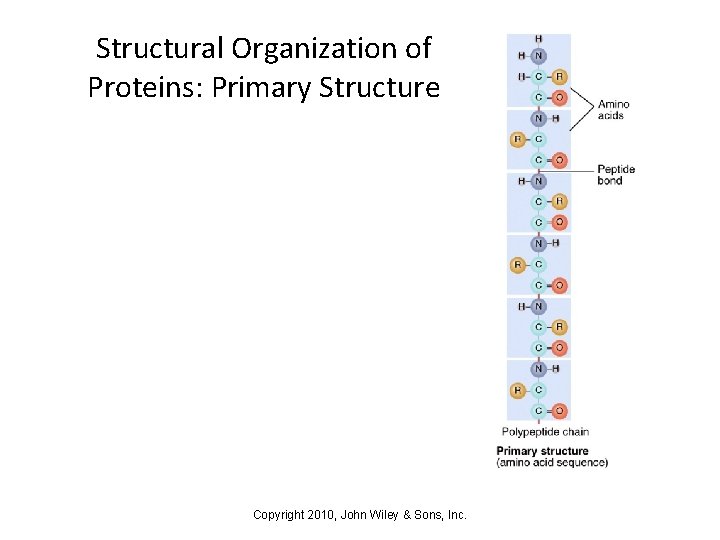
Structural Organization of Proteins: Primary Structure Copyright 2010, John Wiley & Sons, Inc.

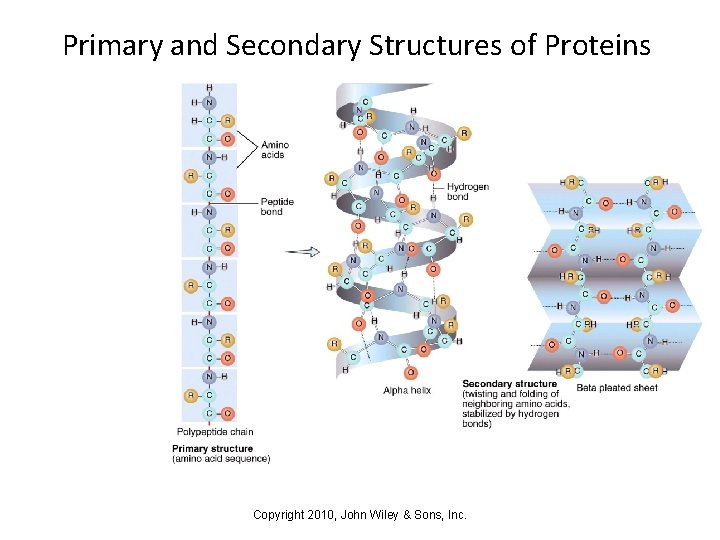
Primary and Secondary Structures of Proteins Copyright 2010, John Wiley & Sons, Inc.

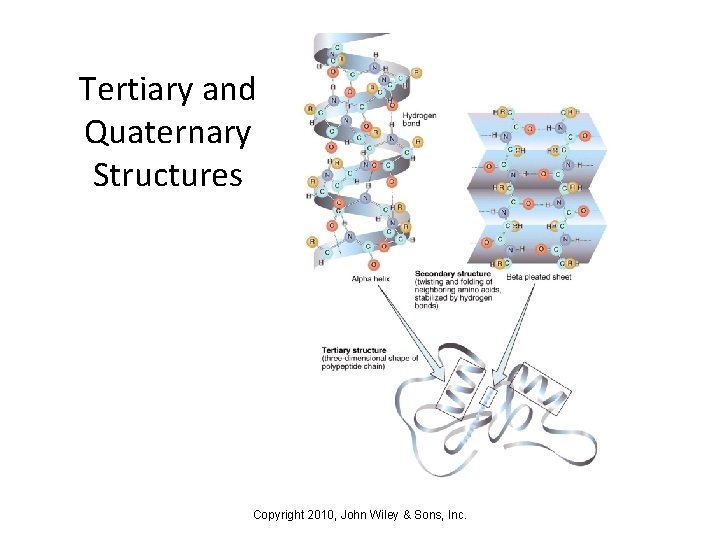
Tertiary and Quaternary Structures Copyright 2010, John Wiley & Sons, Inc.

Structural Organization of Proteins: Quaternary Structure Copyright 2010, John Wiley & Sons, Inc.

Enzymes • Enzymes are protein catalysts that speed up chemical reactions • Most reactions in our bodies require an enzyme • Enzymes have three key properties: – Enzymes are specific → only one chemical reaction is affected – Enzymes are efficient → reaction products are produced quickly – Enzymes are regulated by cells in two ways: • The number of enzyme molecules can be adjusted as needed • The activity of each individual enzyme molecule can be controlled Copyright 2010, John Wiley & Sons, Inc.

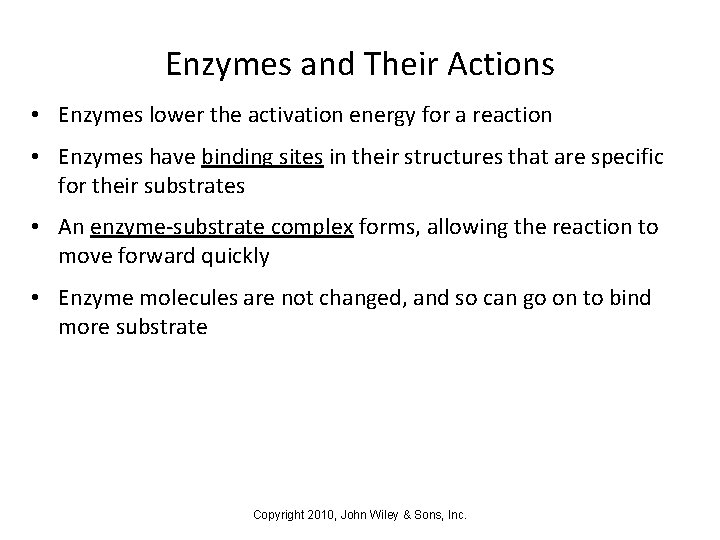
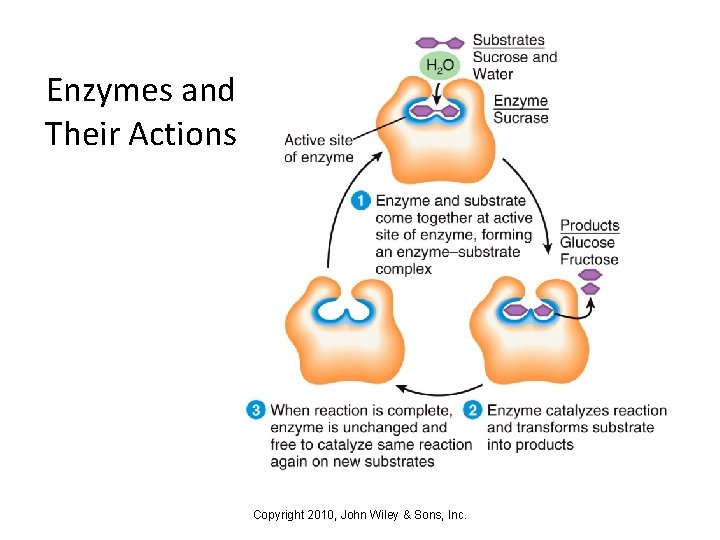
Enzymes and Their Actions • Enzymes lower the activation energy for a reaction • Enzymes have binding sites in their structures that are specific for their substrates • An enzyme-substrate complex forms, allowing the reaction to move forward quickly • Enzyme molecules are not changed, and so can go on to bind more substrate Copyright 2010, John Wiley & Sons, Inc.

Enzymes and Their Actions Copyright 2010, John Wiley & Sons, Inc.

Enzymes Interactions Animation • Enzyme Functions and ATP You must be connected to the internet to run this animation. Copyright 2010, John Wiley & Sons, Inc.

Nucleic Acids are Polymers of Nucleotides • Nucleic acids play central roles in the storage of genetic information and in the synthesis of proteins • Each nucleotide contains: a nitogenous base, a five-carbon sugar, and a PO 4 Copyright 2010, John Wiley & Sons, Inc.

Nucleic Acids • • DNA and RNA Nucleotide 5 C sugar, Nitrogen Base, Phosphate group DNA stores and transmits hereditary info RNA protein synthesis

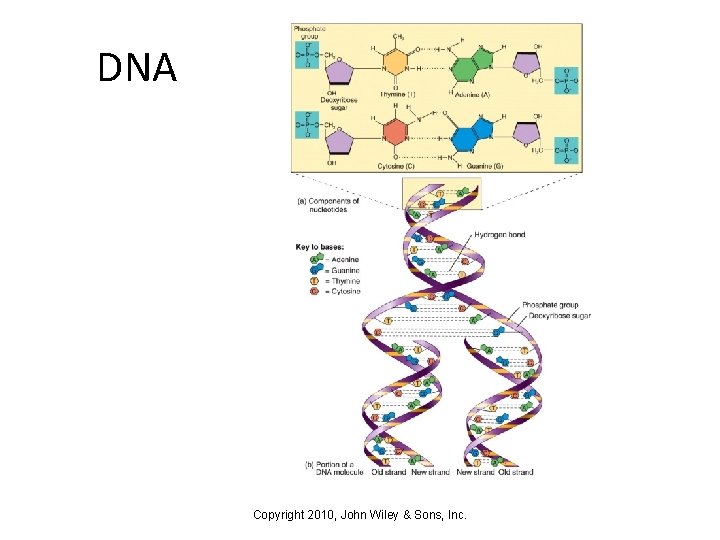
DNA Copyright 2010, John Wiley & Sons, Inc.

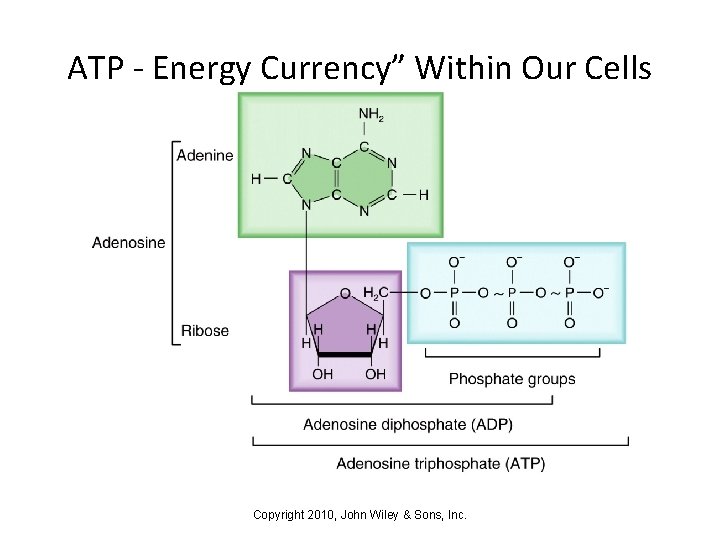
ATP - Energy Currency” Within Our Cells • Adenosine triphosphate is ATP, a nucleotide with 3 bound phosphate groups • ATP “carries” energy within cells, from one chemical reaction to another • Many enzymes use ATP – ATP breakdown releases energy needed by the cell – ATP synthesis requires energy from other sources Copyright 2010, John Wiley & Sons, Inc.

ATP - Energy Currency” Within Our Cells Copyright 2010, John Wiley & Sons, Inc.

ATP Interactions Animation • Enzyme Functions and ATP You must be connected to the internet to run this animation. Copyright 2010, John Wiley & Sons, Inc.