The Chemical Basis of Life Organic Compounds Compounds

The Chemical Basis of Life

Organic Compounds Ø Compounds containing carbon Ø (Actually contain carbon, hydrogen, and oxygen) Ø Compounds that come from living things

Q 1)Give two examples of organic compounds? Ø Sugar, Starch

Inorganic compounds Ø Don’t contain carbon Ø Don’t come from living things Ø Exceptions: monoxide Carbon dioxide, Carbon

Q 2) Give 2 examples of inorganic molecules Ø Water, Salt
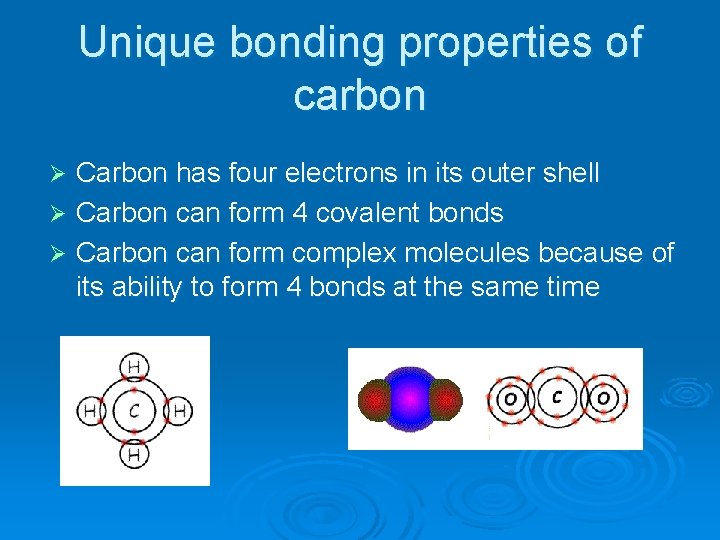
Unique bonding properties of carbon Carbon has four electrons in its outer shell Ø Carbon can form 4 covalent bonds Ø Carbon can form complex molecules because of its ability to form 4 bonds at the same time Ø
Chemistry of carbon Ø Carbon can form l Single covalent bonds • Shares 1 electron with one other atom. l Double covalent bonds • Shares 2 electrons with one other atom l Triple covalent bonds (rare) • Shares 3 electrons with one other atom (See examples of these bonds on the bottom of page 49)
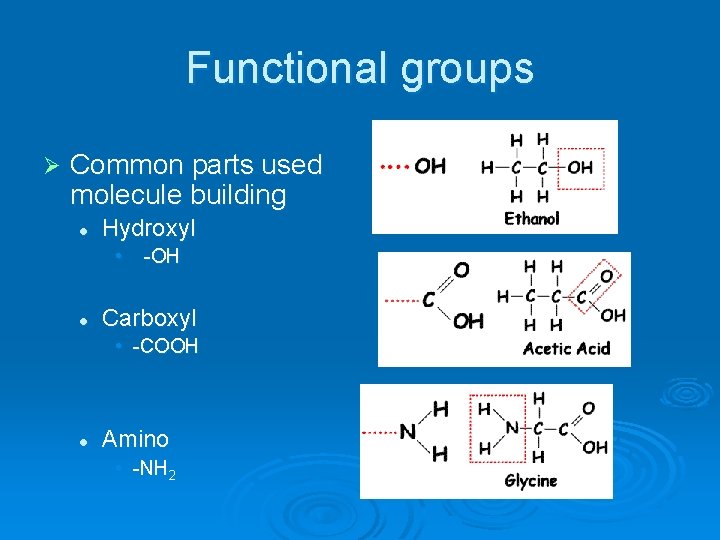
Functional groups Ø Common parts used molecule building l Hydroxyl • l -OH Carboxyl • -COOH l Amino • -NH 2
Monomers Ø Simple building block molecules
Polymers Ø Two or more monomers covalently bonded together. Ø Can be two or two thousand… Ø Allow very large molecules to built with only a few basic parts.

Two chemical reactions used Ø Dehydration Synthesis l l Covalent bond is formed by the removal of water. Two monomers become joined together.
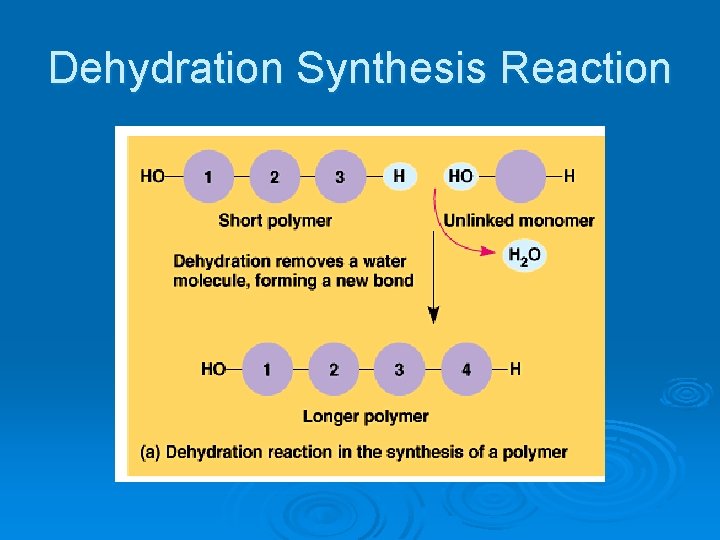
Dehydration Synthesis Reaction

Two Chemical Reactions Used Ø Hydrolysis l Separation of two monomers by adding water and breaking the covalent bond
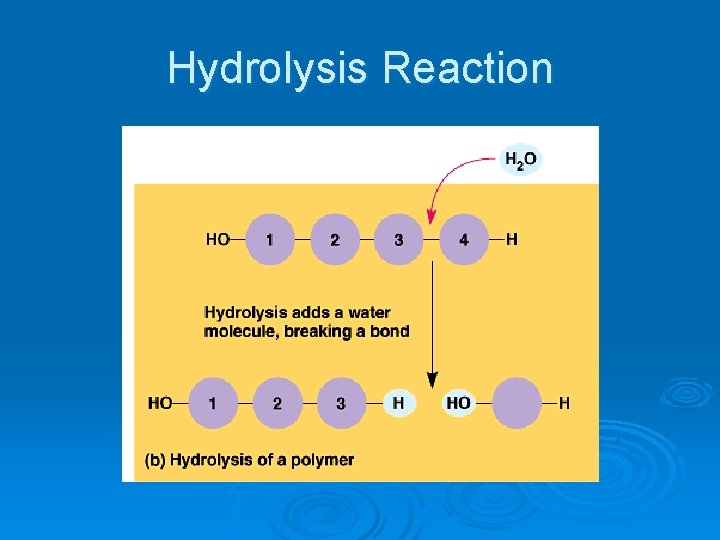
Hydrolysis Reaction

Carbohydrates Ø Made from glucose molecules (sugars) Ø Carbohydrates are used by living things as a source of energy.
Monosaccharides Ø Simple sugars l Mono = one Saccharide = sugar Ø Have the formula C 6 H 12 O 6 Ø Form rings when in water
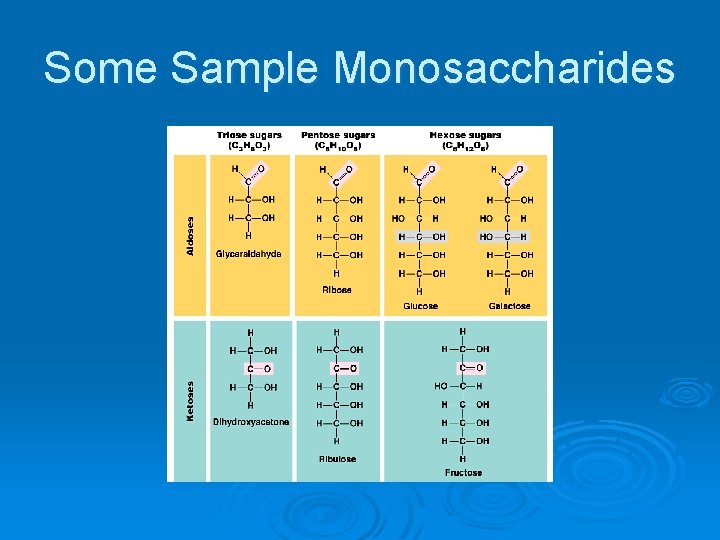
Some Sample Monosaccharides

Q 2) Where do people get glucose molecules? Ø Plants produce glucose during photosynthesis and animals get glucose by eating plants.
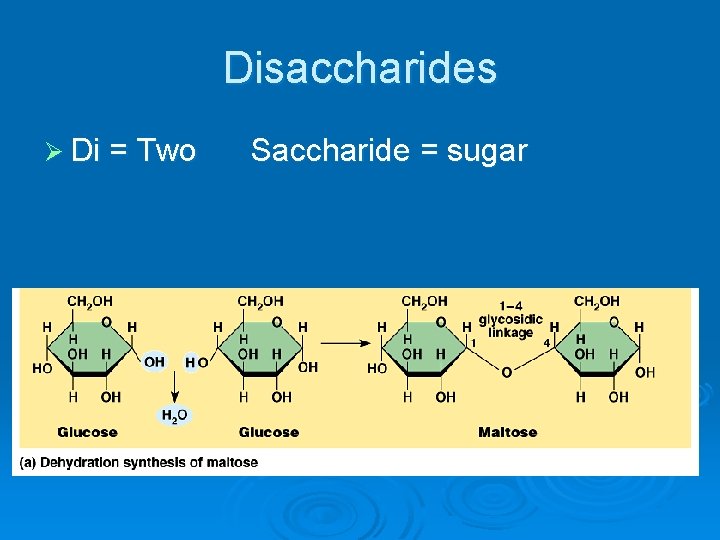
Disaccharides Ø Di = Two Saccharide = sugar
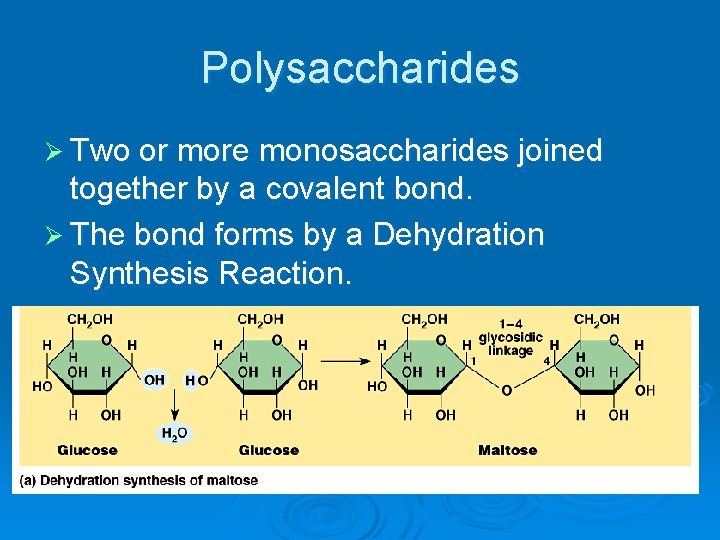
Polysaccharides Ø Two or more monosaccharides joined together by a covalent bond. Ø The bond forms by a Dehydration Synthesis Reaction.

Four types of polysaccharides Made of Glucose
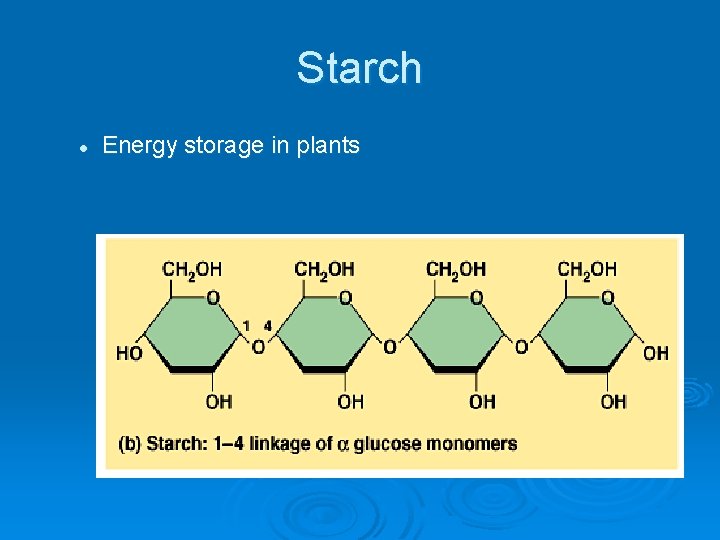
Starch l Energy storage in plants
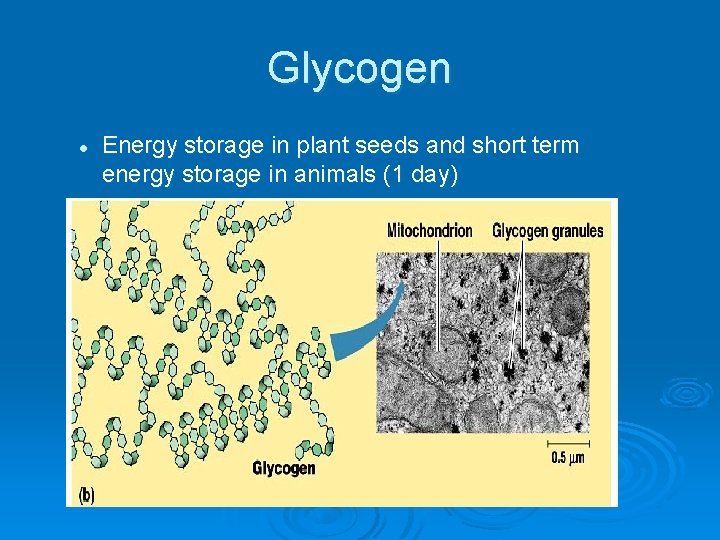
Glycogen l Energy storage in plant seeds and short term energy storage in animals (1 day)
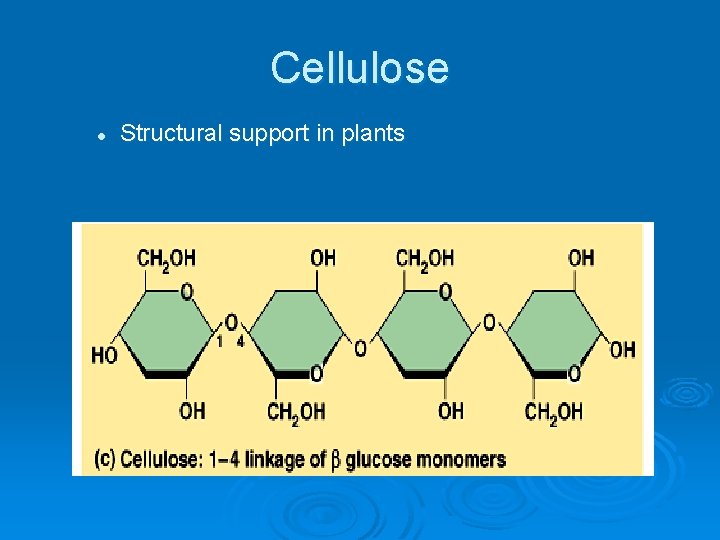
Cellulose l Structural support in plants
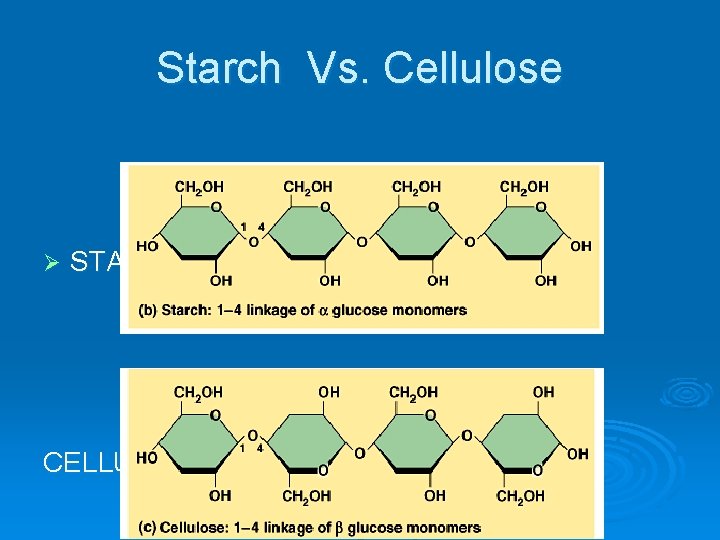
Starch Vs. Cellulose Ø STARCH CELLULOSE

Chitin Ø Used in insect exoskeletons for structural support Ø Harvested and used as surgical stitches

Lipids Ø Fats Ø Oils Ø Waxes Ø Do not dissolve in water!!!
Molecules made from lipids Ø Fats l Energy storage in animals and plant seeds • A gram of fat stores more than twice as much energy as a gram of a polysaccharide.
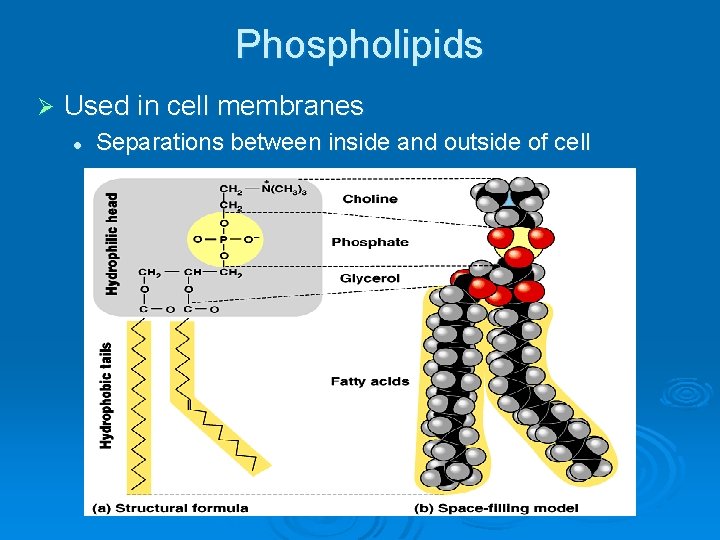
Phospholipids Ø Used in cell membranes l Separations between inside and outside of cell

Waxes Ø Water proof molecules, many uses Ø Example: Waxy coating on leaves prevents water loss
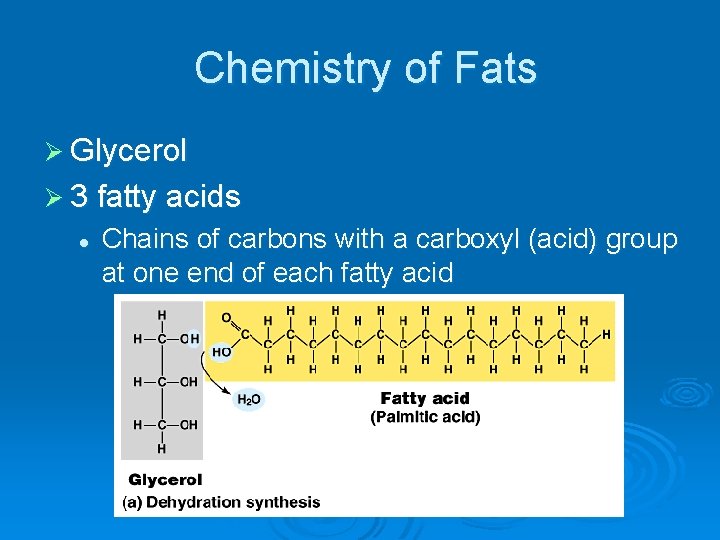
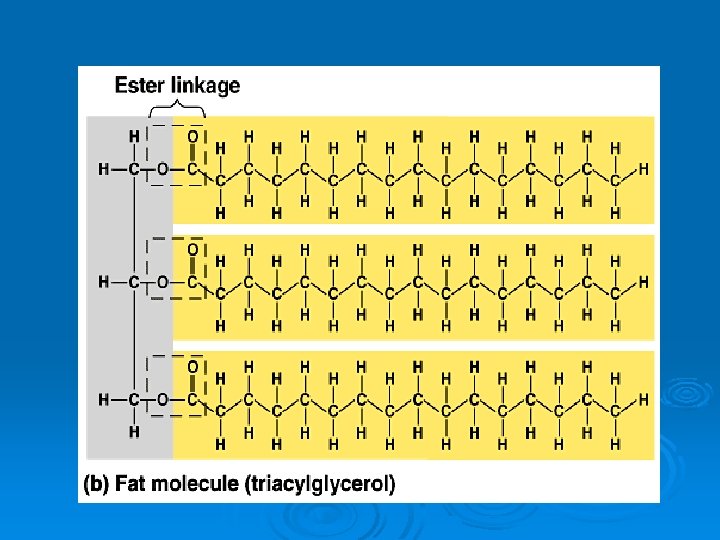
Chemistry of Fats Ø Glycerol Ø 3 fatty acids l Chains of carbons with a carboxyl (acid) group at one end of each fatty acid
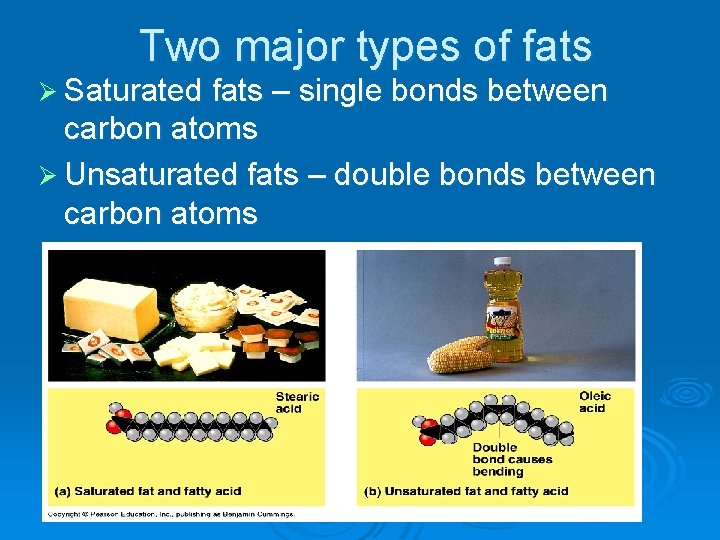
Two major types of fats Ø Saturated fats – single bonds between carbon atoms Ø Unsaturated fats – double bonds between carbon atoms

Q 3) Which type of fat is unhealthy? Ø Saturated fats

What is the difference between fats and oils? Ø Fats are solid at room temperature and oils are liquids at room temperature.
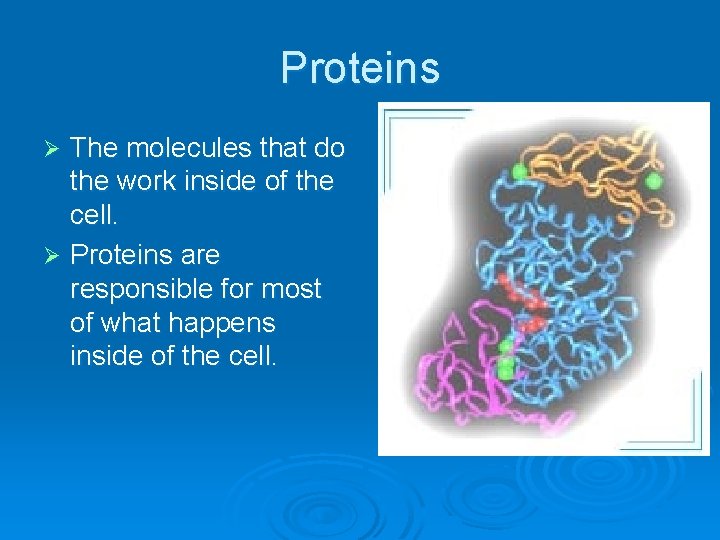
Proteins The molecules that do the work inside of the cell. Ø Proteins are responsible for most of what happens inside of the cell. Ø
Functions of a protein Ø Movement Ø Structural support Ø Storage Ø Defense Ø Regulation of chemical processes

What are two examples of things made of protein? Ø Enzymes (thousands of different types) l Speed up chemical reactions Ø Hemoglobin l Used in red blood cells to transport oxygen
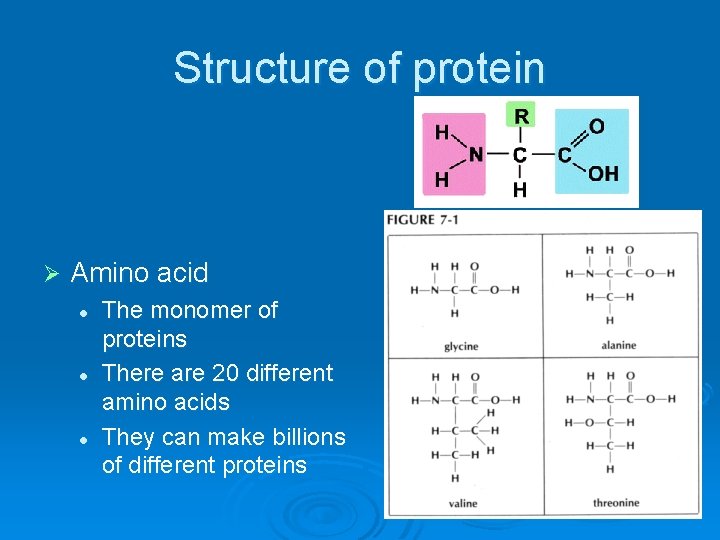
Structure of protein Ø Amino acid l l l The monomer of proteins There are 20 different amino acids They can make billions of different proteins
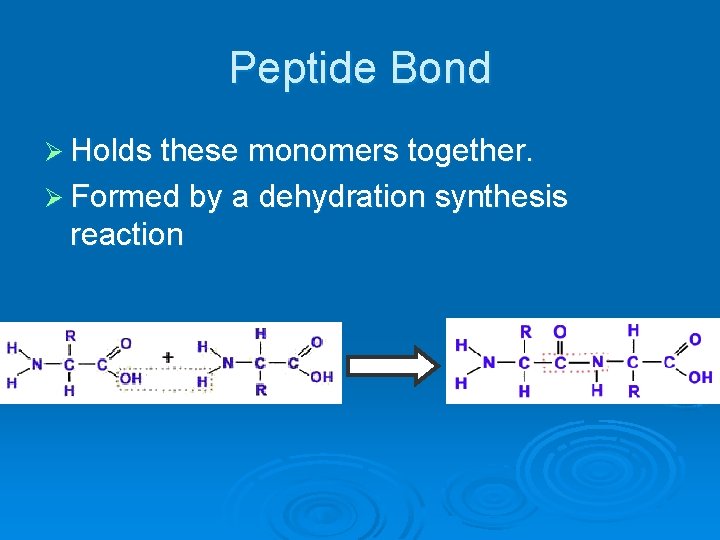
Peptide Bond Ø Holds these monomers together. Ø Formed by a dehydration synthesis reaction
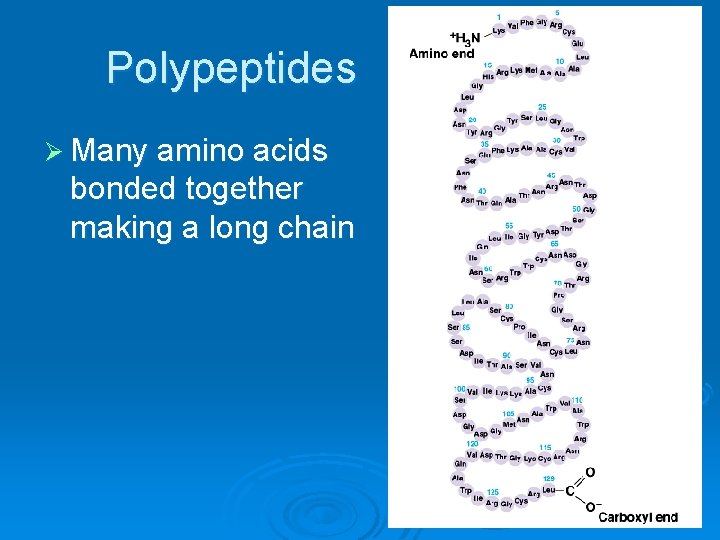
Polypeptides Ø Many amino acids bonded together making a long chain
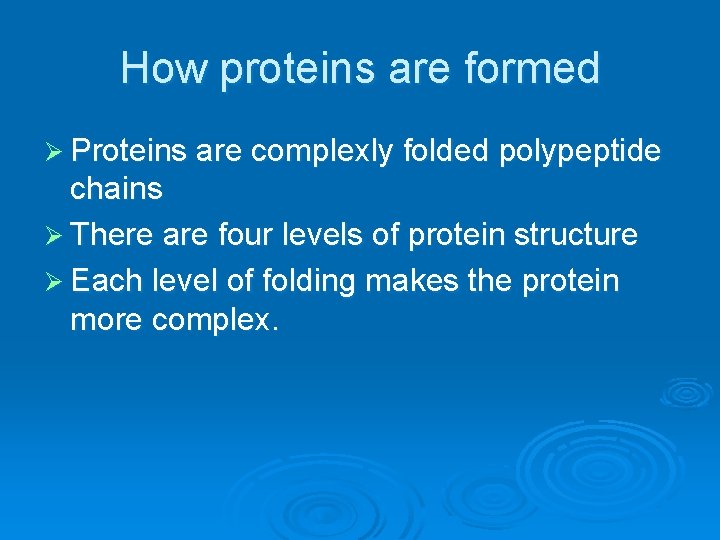
How proteins are formed Ø Proteins are complexly folded polypeptide chains Ø There are four levels of protein structure Ø Each level of folding makes the protein more complex.

One change can be devastating

Stop for today.
The function of enzymes Ø Enzymes are Protein Catalysts l l Increase the speed of chemical reactions without being used up themselves. NOT CHANGED BY REACTION

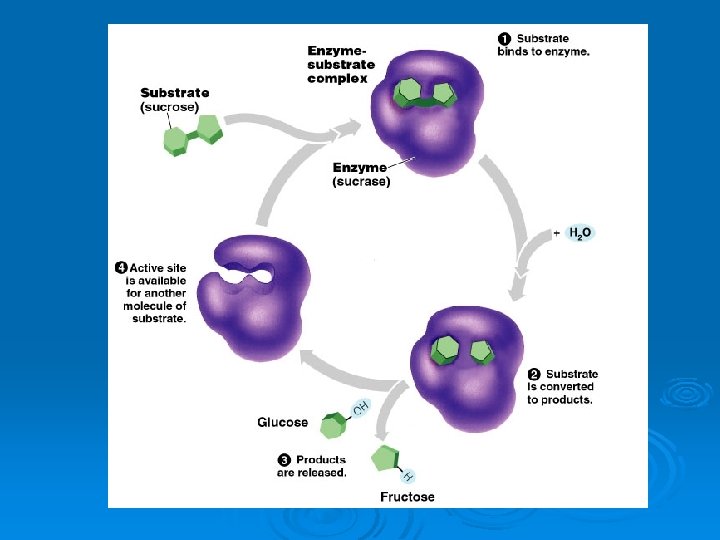
Substrates Ø The molecule that binds to the enzyme Ø These are the ones changed in the reaction
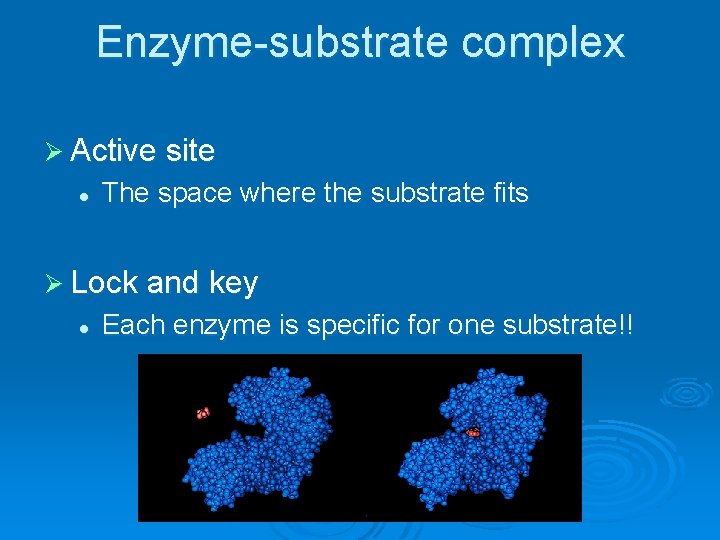
Enzyme-substrate complex Ø Active site l The space where the substrate fits Ø Lock and key l Each enzyme is specific for one substrate!!
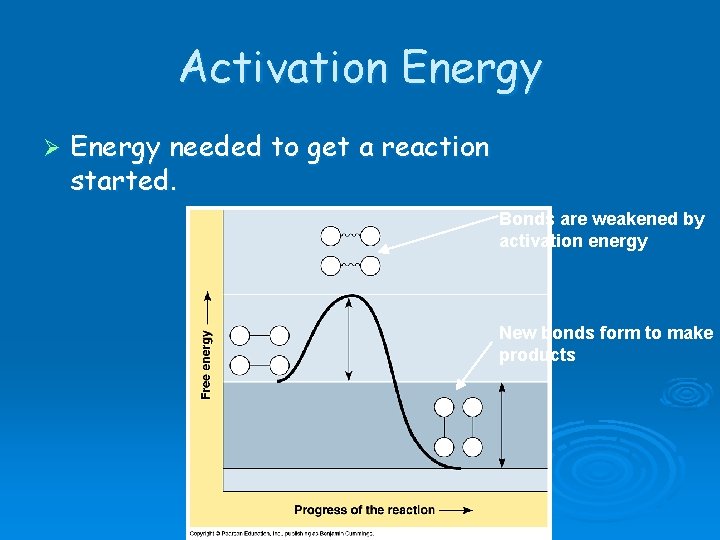
Activation Energy Ø Energy needed to get a reaction started. Bonds are weakened by activation energy New bonds form to make products
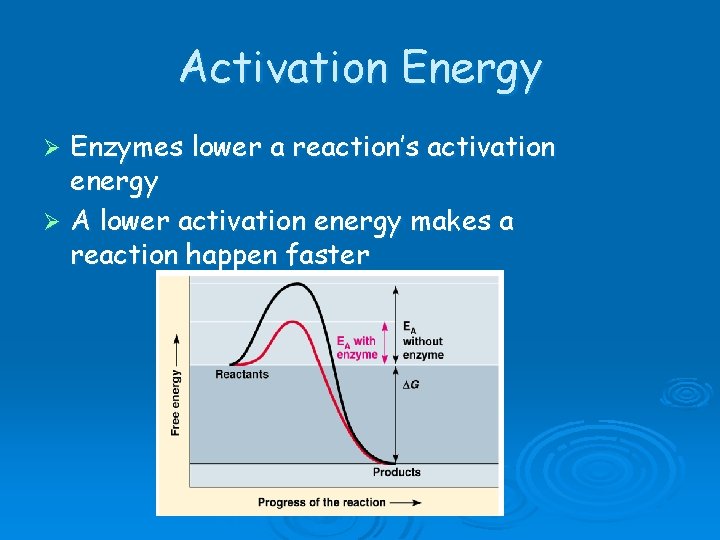
Activation Energy Enzymes lower a reaction’s activation energy Ø A lower activation energy makes a reaction happen faster Ø
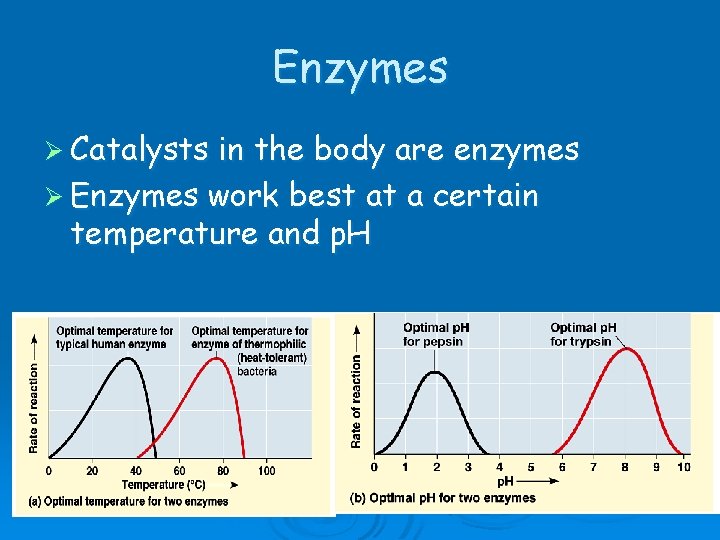
Enzymes Ø Catalysts in the body are enzymes Ø Enzymes work best at a certain temperature and p. H

If the temperature or p. H changes, the enzyme may not function. Ø If the bonds that hold the enzyme’s shape are changed, the enzyme will come apart. Ø If this happens, the enzyme will denature.
Nucleic Acids Ø Polymers which are used to store genetic information

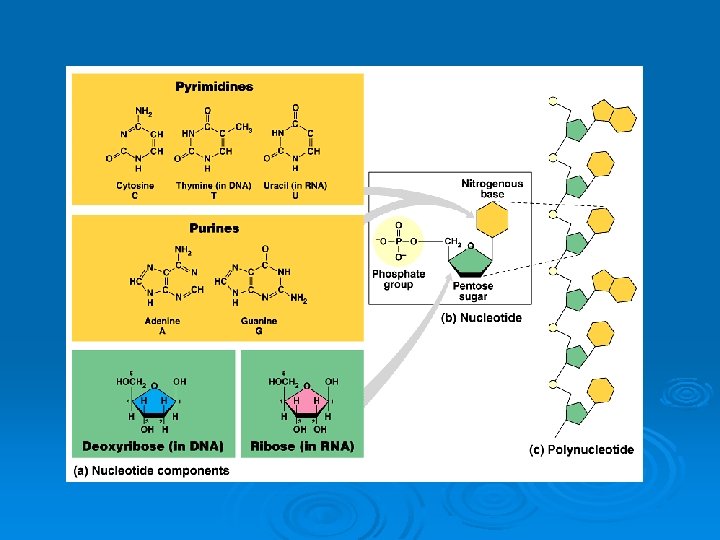
Nucleotide Ø Monomer of nucleic acids Ø Made from • • • 1 sugar 1 base 1 phosphate
Two types of nucleic acids and their uses: Ø Deoxyribonucleic Acid l l DNA Stores genetic information and passes it on to the next generation Ø Ribonucleic Acid l l RNA Takes information and uses it to make proteins
The information is stored in bases Ø The differences in the nucleotides is in the bases. Ø The order of these bases makes up the genetic CODE.

DNA Bases Ø There are four bases used in DNA Cytosine (C), Thymine (T) Adenine (A), Guanine (G)
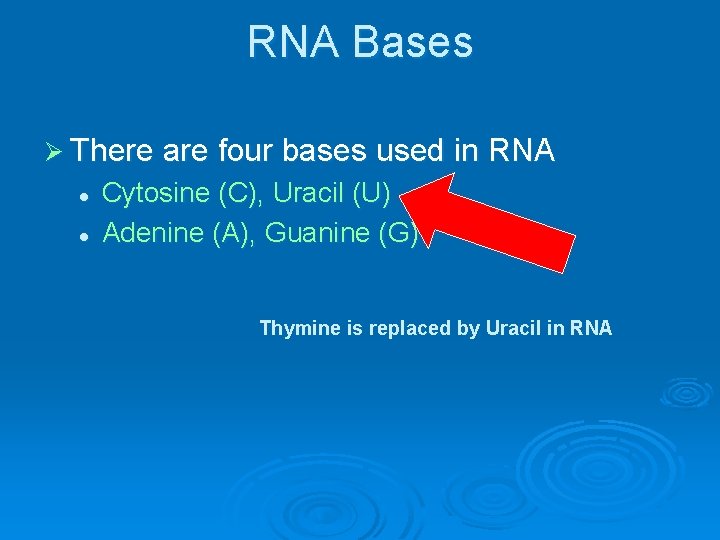
RNA Bases Ø There are four bases used in RNA l l Cytosine (C), Uracil (U) Adenine (A), Guanine (G) Thymine is replaced by Uracil in RNA
- Slides: 60