The Cell Cycle Phases include 1 Interphase Preparation



















- Slides: 44




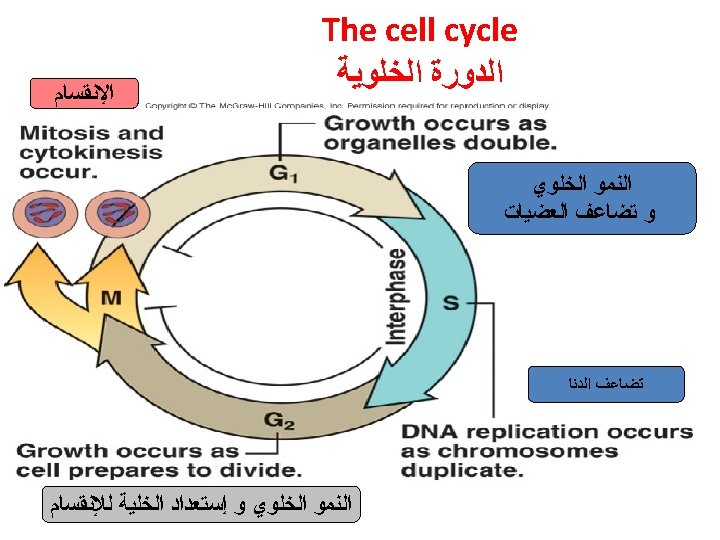
The Cell Cycle Phases include: 1. Interphase – Preparation phases for mitosis 2. Mitosis – Cell division or splitting Interphase 1. G 1 (Growth) 2. S 3. G 2 (Growth)

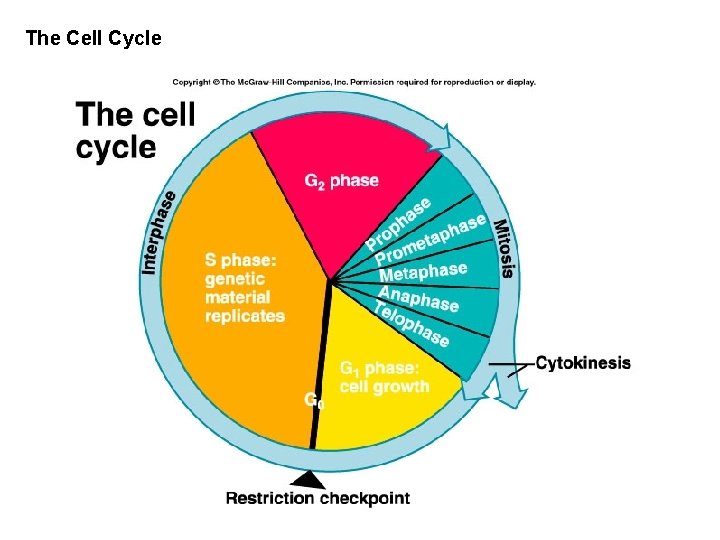
The Cell Cycle

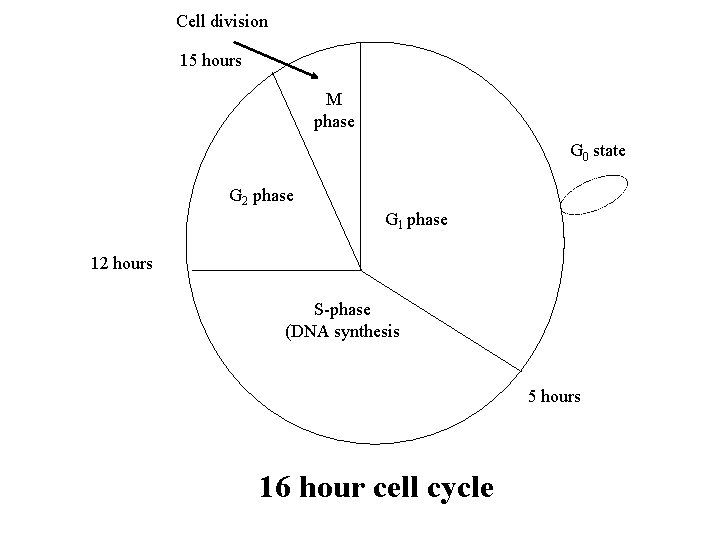
Cell division 15 hours M phase G 0 state G 2 phase G 1 phase 12 hours S-phase (DNA synthesis 5 hours 16 hour cell cycle


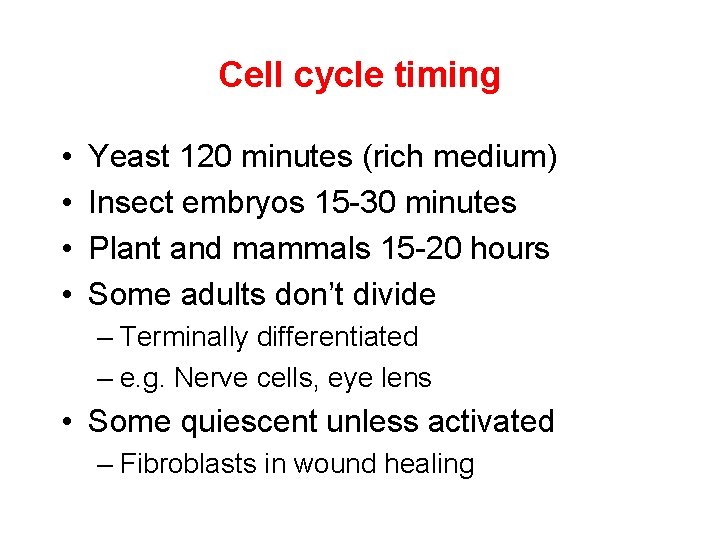
Cell cycle timing • • Yeast 120 minutes (rich medium) Insect embryos 15 -30 minutes Plant and mammals 15 -20 hours Some adults don’t divide – Terminally differentiated – e. g. Nerve cells, eye lens • Some quiescent unless activated – Fibroblasts in wound healing

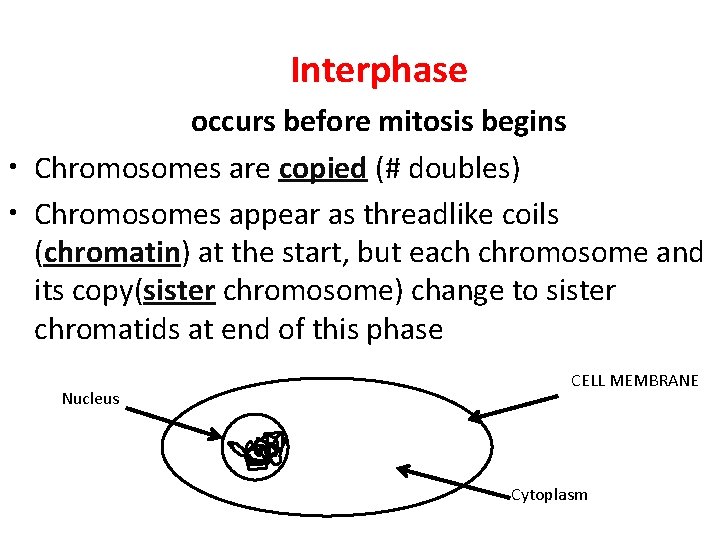
Interphase occurs before mitosis begins • Chromosomes are copied (# doubles) • Chromosomes appear as threadlike coils (chromatin) at the start, but each chromosome and its copy(sister chromosome) change to sister chromatids at end of this phase Nucleus CELL MEMBRANE Cytoplasm


• G 1 phase – Cell checks everything OK for DNA replication – Accumulates signals that activate replication – Chloroplast and mitochondria division not linked to cell cycle


• S-phase – The chromosomes replicate – Two daughter chromosomes are called chromatids – Joined at centromere – Number of chromosomes in diploid is four


• G 2 -phase – Cell checks everything is OK for cell division – Accumulates proteins that activate cell division


Why have a cell cycle? • Comprises gaps and distinct phases of DNA replication and cell division • If replicating DNA is forced to condense (as in mitosis) they fragment • Similarly if replication before mitosis – Unequal genetic seperation • Important to keep DNA replication and mitosis separate

• Important to have divisions in mitosis • e. g. Important metaphase complete before anaphase. • If not segregation of chromosomes before attachment of chromatids to microtubles in opposite poles is possible • Down syndrome due to extra chromosome 21

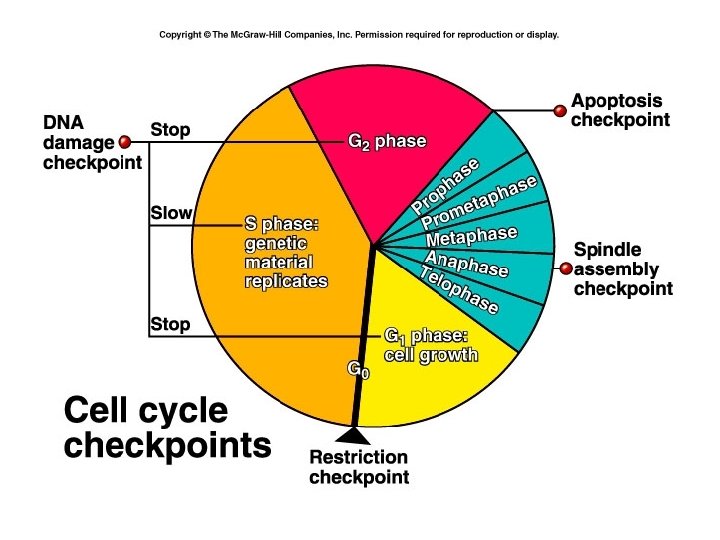
• Gaps provide cell with chance to assess its status prior to DNA replication or cell division • During the cell cycle there are several checks to monitor status • These are called checkpoints


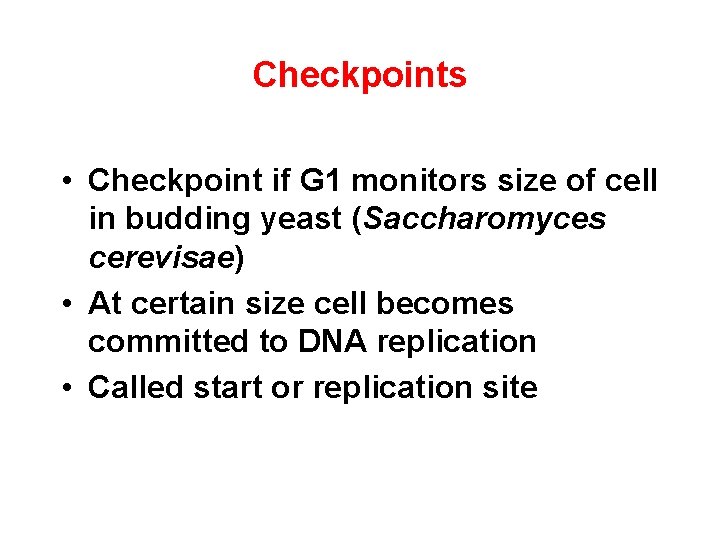
Checkpoints • Checkpoint if G 1 monitors size of cell in budding yeast (Saccharomyces cerevisae) • At certain size cell becomes committed to DNA replication • Called start or replication site

Evidence of size checkpoint • Yeast cells (budding yeast) grown in rich medium • Switch to minimal medium • Cells recently entering G 1 (buds) delayed in G 1 (longer to enter S-phase) • Large cells above threshold size still go to S-phase at same time as in rich medium

Evidence of size checkpoint • Yeast in rich medium – 120 minute cell cycle • Short G 1 phase • Yeast in minimal medium – Eight hour cell cycle primarily because of long G 1 phase

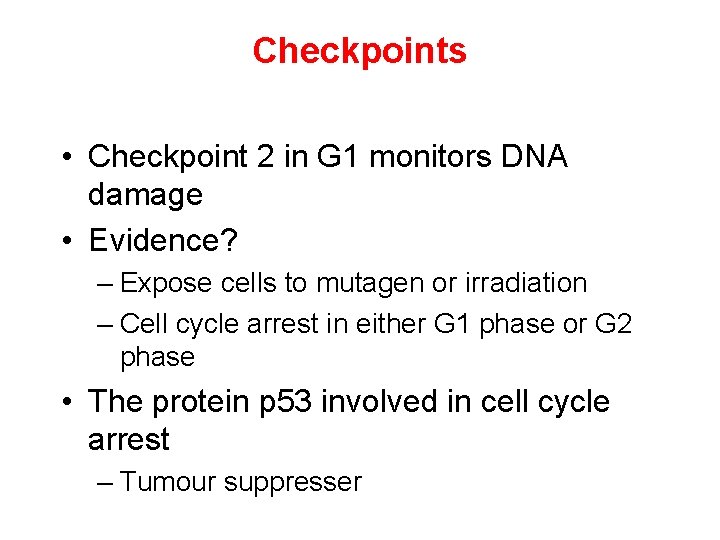
Checkpoints • Checkpoint 2 in G 1 monitors DNA damage • Evidence? – Expose cells to mutagen or irradiation – Cell cycle arrest in either G 1 phase or G 2 phase • The protein p 53 involved in cell cycle arrest – Tumour suppresser

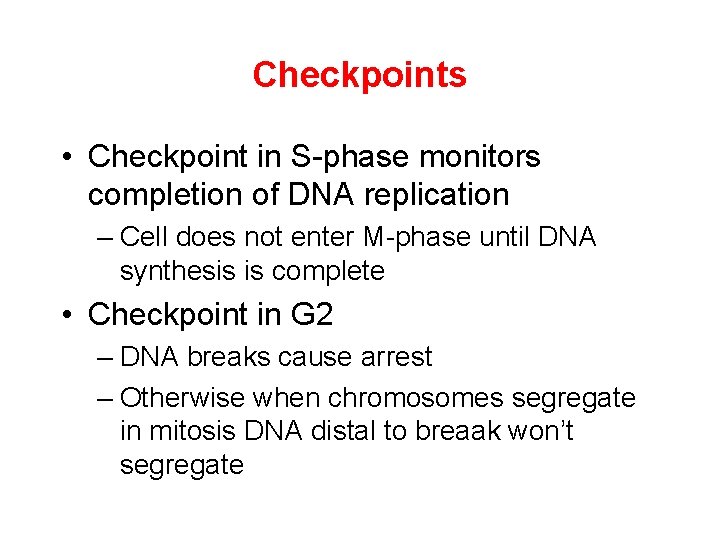
Checkpoints • Checkpoint in S-phase monitors completion of DNA replication – Cell does not enter M-phase until DNA synthesis is complete • Checkpoint in G 2 – DNA breaks cause arrest – Otherwise when chromosomes segregate in mitosis DNA distal to breaak won’t segregate

Checkpoints • Checkpoint in mitosis – Senses when mitotic spindles have not formed – Arrests in M-phase – Otherwise unequal segregation of chromosomes into daughter cells • Described cell cycle, now I will talk about genes and proteins that control this process

MPF • Protein identified that causes mitosis • Called maturation promoting factor • MPF in all mitotic cells from yeast to humans • Renamed mitosis-promoting factor

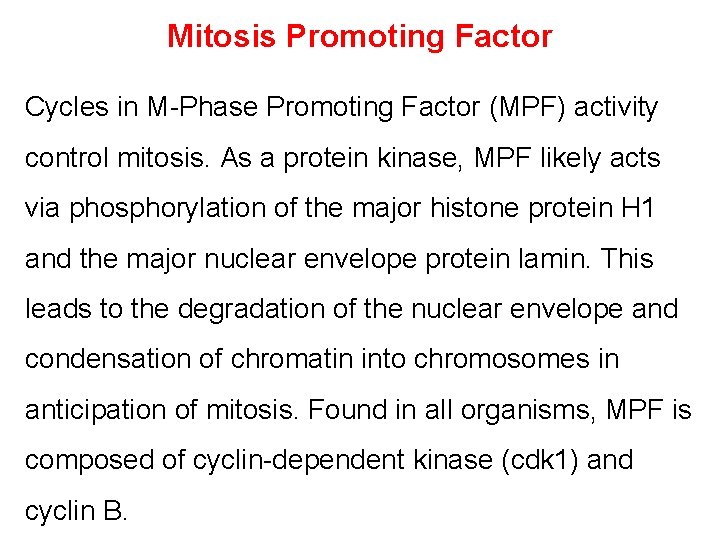
Mitosis Promoting Factor Cycles in M-Phase Promoting Factor (MPF) activity control mitosis. As a protein kinase, MPF likely acts via phosphorylation of the major histone protein H 1 and the major nuclear envelope protein lamin. This leads to the degradation of the nuclear envelope and condensation of chromatin into chromosomes in anticipation of mitosis. Found in all organisms, MPF is composed of cyclin-dependent kinase (cdk 1) and cyclin B.

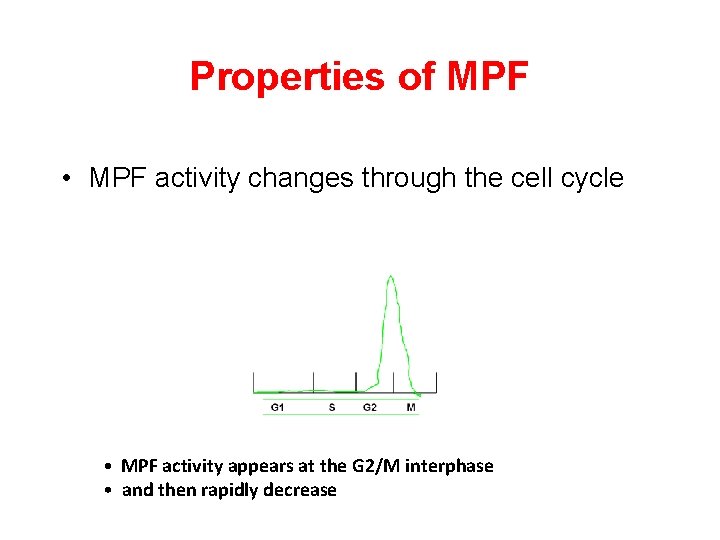
Properties of MPF • MPF activity changes through the cell cycle • MPF activity appears at the G 2/M interphase • and then rapidly decrease

How does MPF cause mitosis? • It’s a protein kinase – Phosphorylates proteins • Phosphorylates proteins involved in mitosis • Phosphorylates histones causing chromatin condensation • Phosphorylates nuclear membrane proteins (lamins) causing membrane disruption

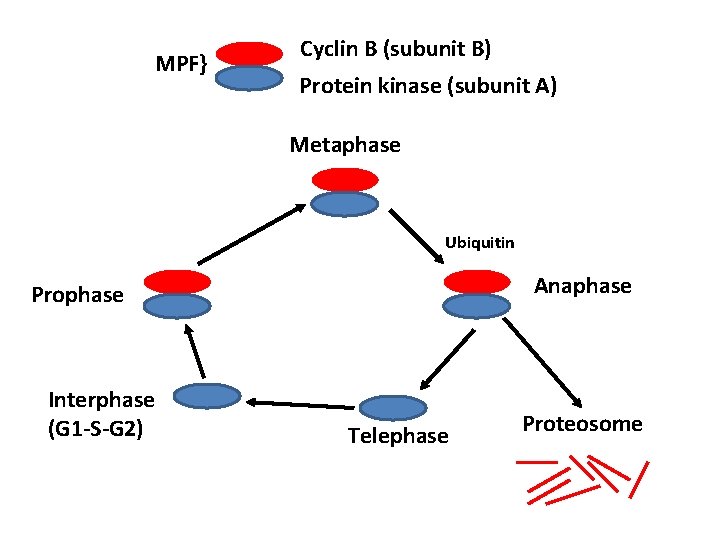
Characterisation of MPF • Consists of two subunits; A and B • Subunit A: Protein kinase • Subunit B: Regulatory polypeptide called cyclin B • Protein kinase present throughout cell cycle • Cyclin B gradually increases during interphase (G 1, S, G 2) • Cyclin B falls abruptly in anaphase (mid -mitosis)

MPF} Cyclin B (subunit B) Protein kinase (subunit A) Metaphase Ubiquitin Anaphase Prophase Interphase (G 1 -S-G 2) Telephase Proteosome

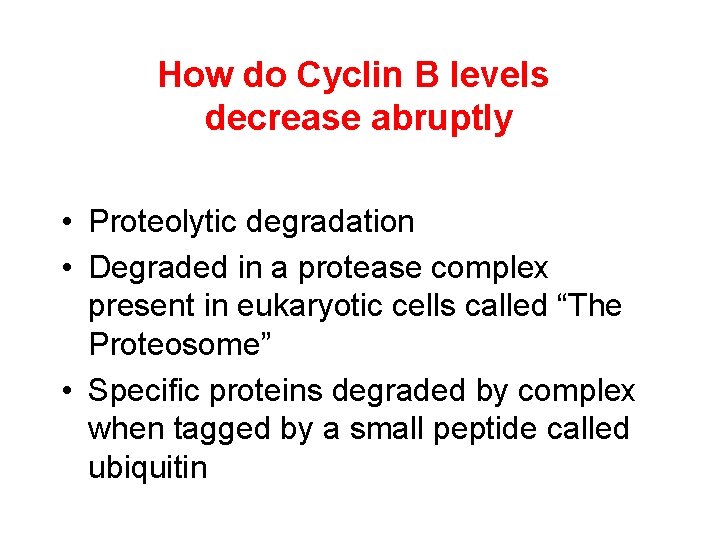
How do Cyclin B levels decrease abruptly • Proteolytic degradation • Degraded in a protease complex present in eukaryotic cells called “The Proteosome” • Specific proteins degraded by complex when tagged by a small peptide called ubiquitin

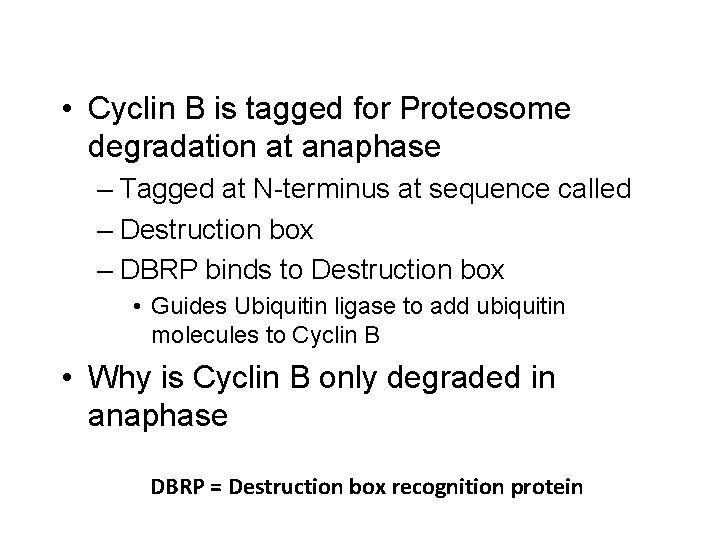
• Cyclin B is tagged for Proteosome degradation at anaphase – Tagged at N-terminus at sequence called – Destruction box – DBRP binds to Destruction box • Guides Ubiquitin ligase to add ubiquitin molecules to Cyclin B • Why is Cyclin B only degraded in anaphase DBRP = Destruction box recognition protein

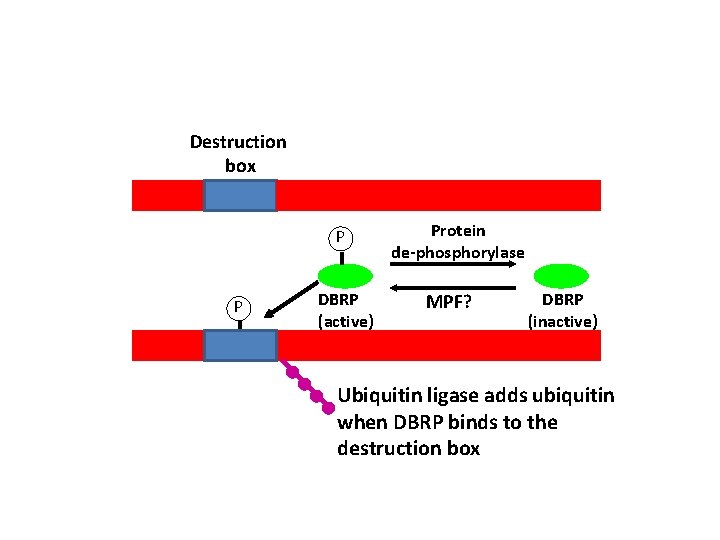
Destruction box P P DBRP (active) Protein de-phosphorylase MPF? DBRP (inactive) Ubiquitin ligase adds ubiquitin when DBRP binds to the destruction box

• DBRP is normally inactive and is only activated in anaphase via phosphorylation • Possible MPF phosphorylates DBRP causing Cyclin B destruction – Binds to the destruction box – Activates ubiquitin ligase to add ubiquitin to Cyclin B – Cyclin B then targeted to the Proteosome for degradation

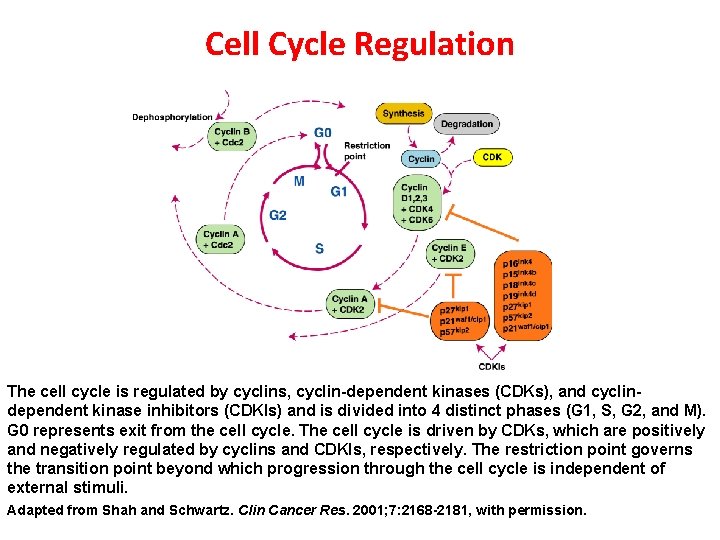
Cell Cycle Regulation The cell cycle is regulated by cyclins, cyclin-dependent kinases (CDKs), and cyclindependent kinase inhibitors (CDKIs) and is divided into 4 distinct phases (G 1, S, G 2, and M). G 0 represents exit from the cell cycle. The cell cycle is driven by CDKs, which are positively and negatively regulated by cyclins and CDKIs, respectively. The restriction point governs the transition point beyond which progression through the cell cycle is independent of external stimuli. Adapted from Shah and Schwartz. Clin Cancer Res. 2001; 7: 2168 -2181, with permission.

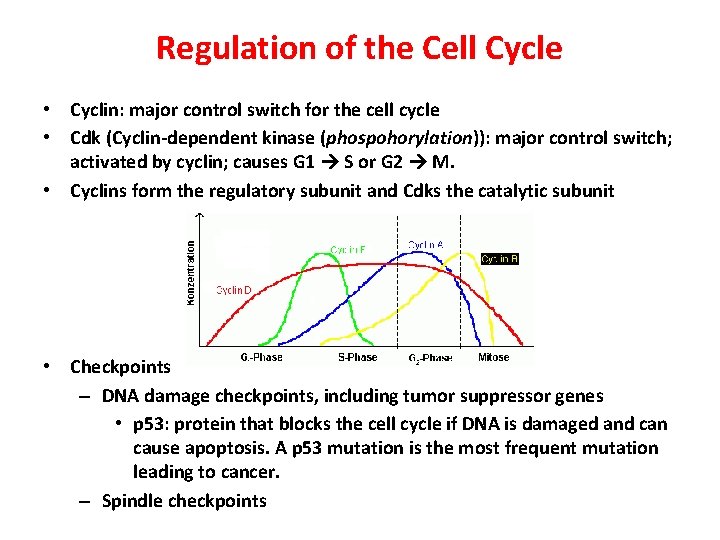
Regulation of the Cell Cycle • Cyclin: major control switch for the cell cycle • Cdk (Cyclin-dependent kinase (phospohorylation)): major control switch; activated by cyclin; causes G 1 S or G 2 M. • Cyclins form the regulatory subunit and Cdks the catalytic subunit • Checkpoints – DNA damage checkpoints, including tumor suppressor genes • p 53: protein that blocks the cell cycle if DNA is damaged and can cause apoptosis. A p 53 mutation is the most frequent mutation leading to cancer. – Spindle checkpoints

What Are Cyclin-Dependent Kinases? § Of the many proteins involved in cell cycle control, cyclindependent kinases (CDKs) are among the most important. § CDKs are a family of multifunctional enzymes that can modify various protein substrates involved in cell cycle progression. § Specifically, CDKs phosphorylate their substrates by transferring phosphate groups from ATP to specific stretches of amino acids in the substrates. § Different types of eukaryotic cells contain different types and numbers of CDKs. For example, yeast have only a single CDK, whereas vertebrates have four different ones.

• As their name suggests, CDKs require the presence of cyclins to become active. • Cyclins are a family of proteins that have no enzymatic activity of their own but activate CDKs by binding to them. • CDKs must also be in a particular phosphorylation state — with some sites phosphorylated and others dephosphorylated — in order for activation to occur. • Correct phosphorylation depends on the action of other kinases and a second class of enzymes called phosphatases that are responsible for removing phosphate groups from proteins.

• All eukaryotes have multiple cyclins, each of which acts during a specific stage of the cell cycle. (In organisms with multiple CDKs, each CDK is paired with a specific cyclin. ) • All cyclins are named according to the stage at which they assemble with CDKs. • Common classes of cyclins include G 1 -phase cyclins, G 1/S -phase cyclins, S-phase cyclins, and M-phase cyclins. Mphase cyclins form M-CDK complexes and drive the cell's entry into mitosis; G 1 cyclins form G 1 -CDK complexes and guide the cell's progress through the G 1 phase; and so on. • All CDKs exist in similar amounts throughout the entire cell cycle. In contrast, cyclin manufacture and breakdown varies by stage — with cell cycle progression dependent on the synthesis of new cyclin molecules.


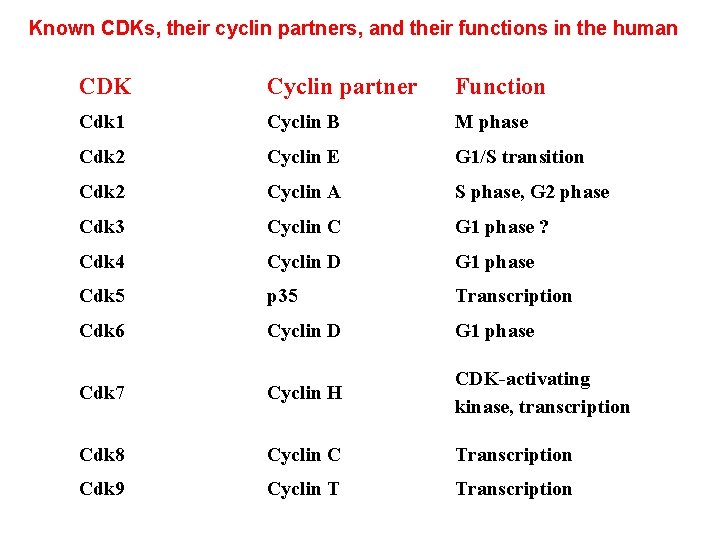
Known CDKs, their cyclin partners, and their functions in the human CDK Cyclin partner Function Cdk 1 Cyclin B M phase Cdk 2 Cyclin E G 1/S transition Cdk 2 Cyclin A S phase, G 2 phase Cdk 3 Cyclin C G 1 phase ? Cdk 4 Cyclin D G 1 phase Cdk 5 p 35 Transcription Cdk 6 Cyclin D G 1 phase Cdk 7 Cyclin H CDK-activating kinase, transcription Cdk 8 Cyclin C Transcription Cdk 9 Cyclin T Transcription

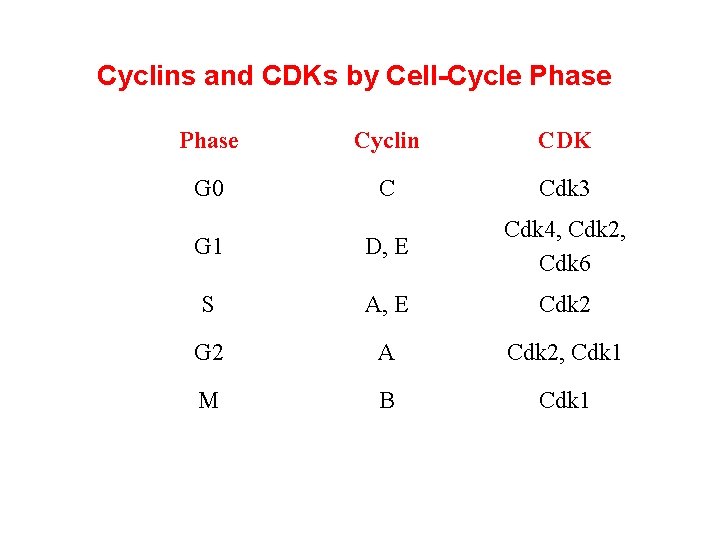
Cyclins and CDKs by Cell-Cycle Phase Cyclin CDK G 0 C Cdk 3 G 1 D, E Cdk 4, Cdk 2, Cdk 6 S A, E Cdk 2 G 2 A Cdk 2, Cdk 1 M B Cdk 1