The Carnot Cycle D Hoult 2011 The product
























- Slides: 48

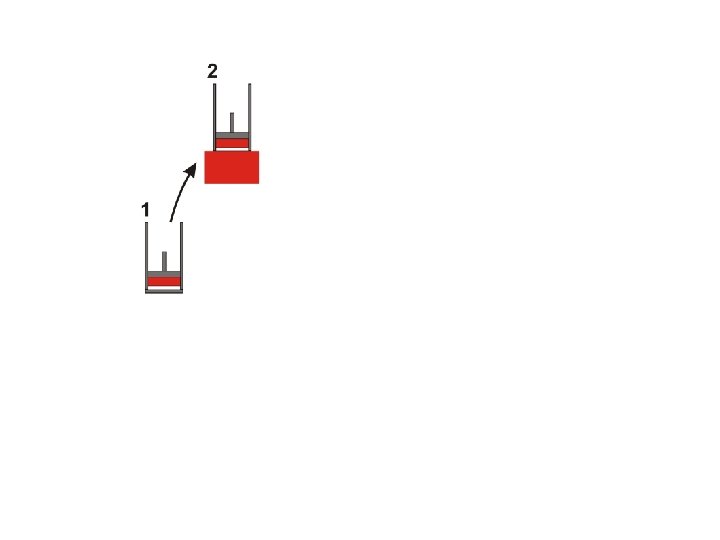
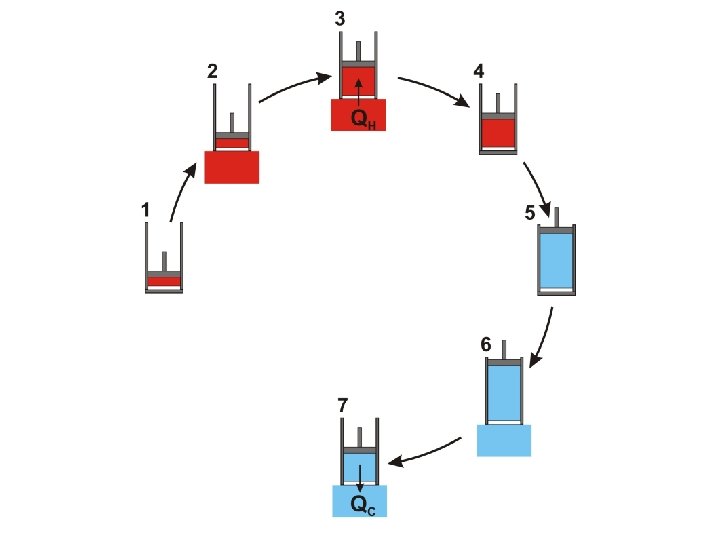
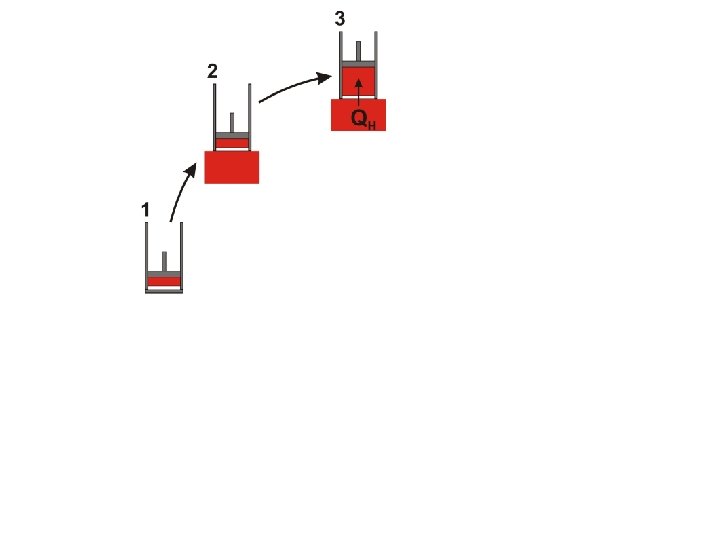
The Carnot Cycle © D Hoult 2011



















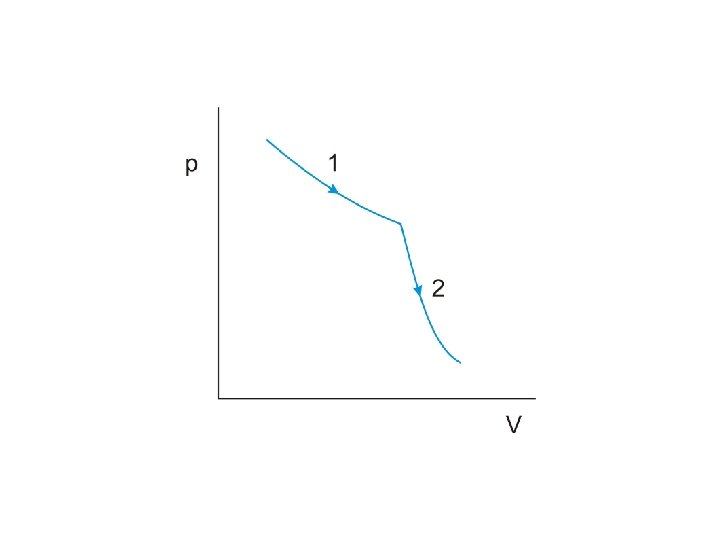
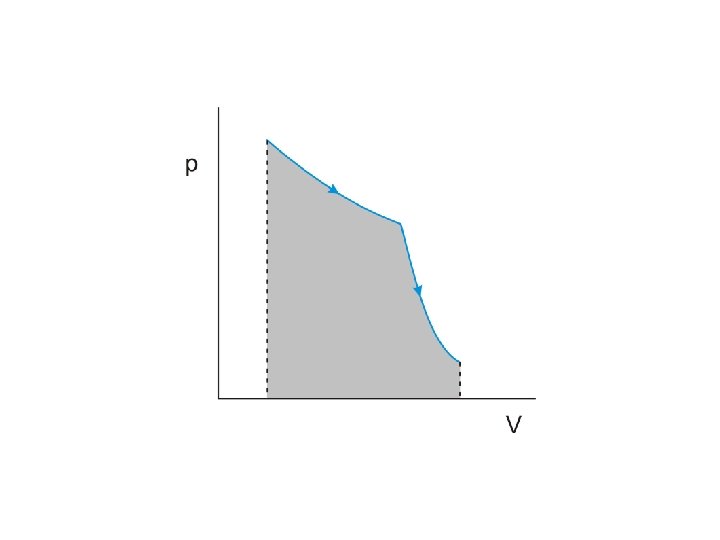
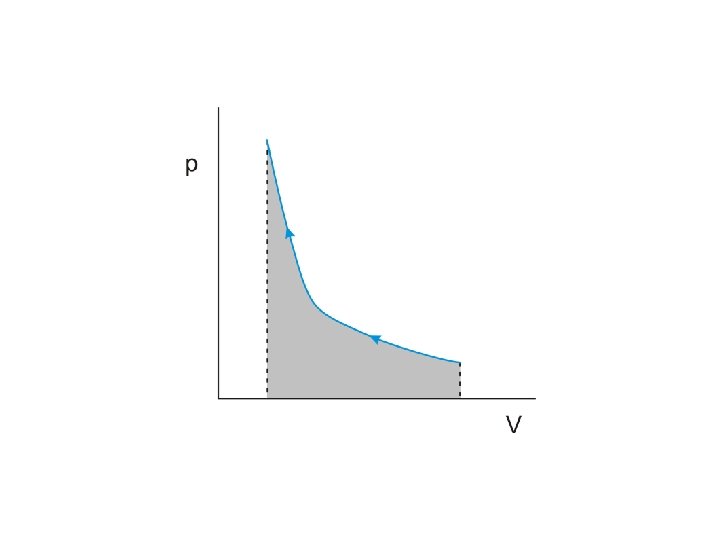
The product of pressure and volume represents a quantity of

The product of pressure and volume represents a quantity of work

The product of pressure and volume represents a quantity of work This is represented by

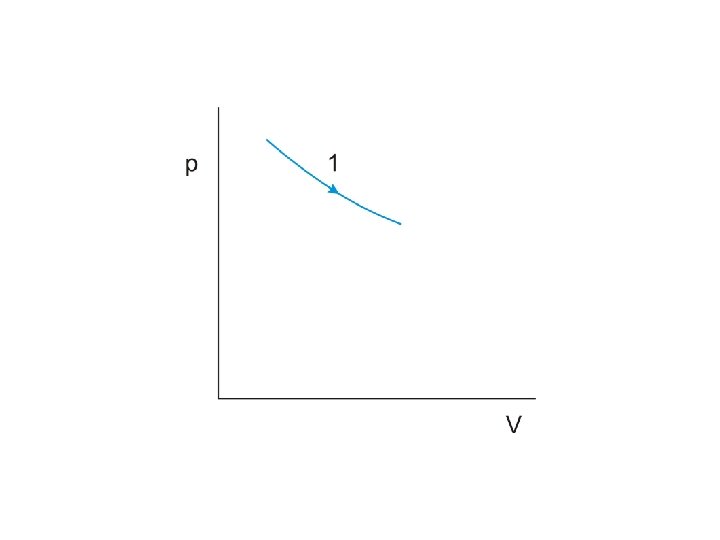
The product of pressure and volume represents a quantity of work This is represented by the area under the curve on the p-V diagram


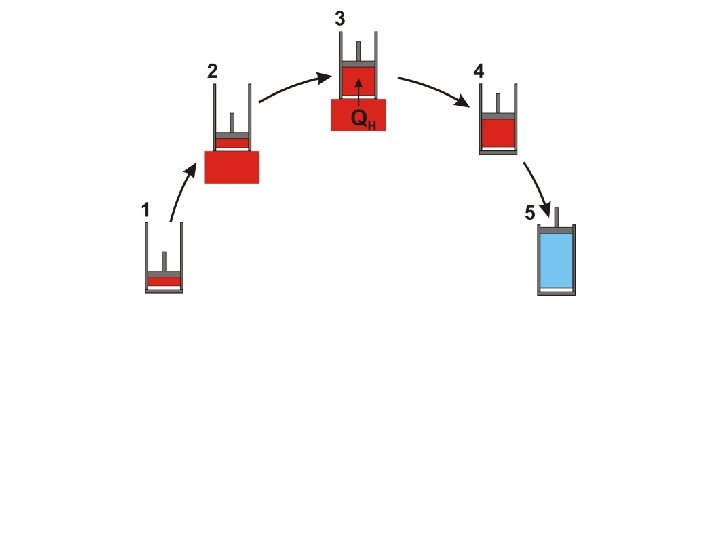
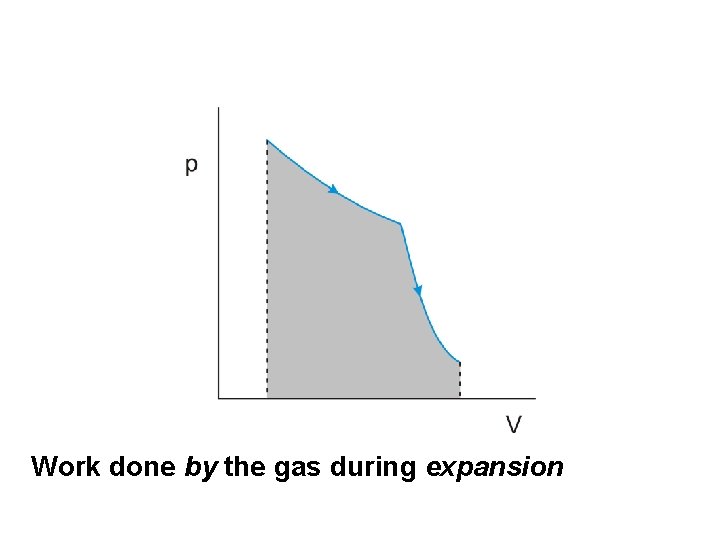
Work done by the gas during expansion


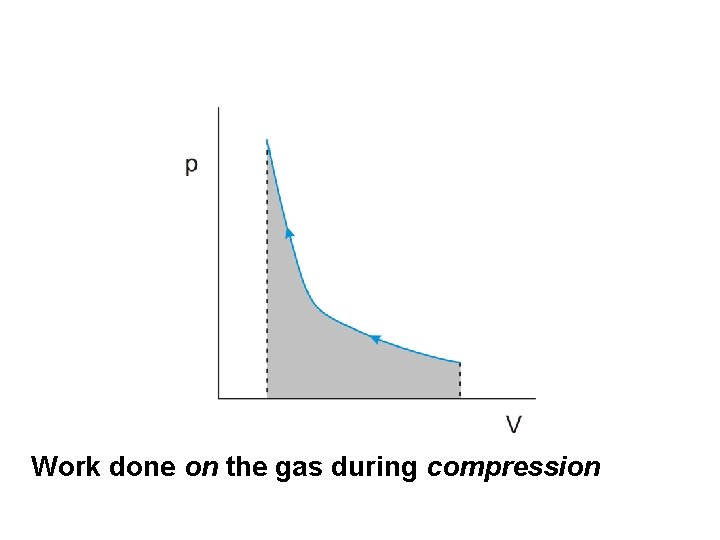
Work done on the gas during compression


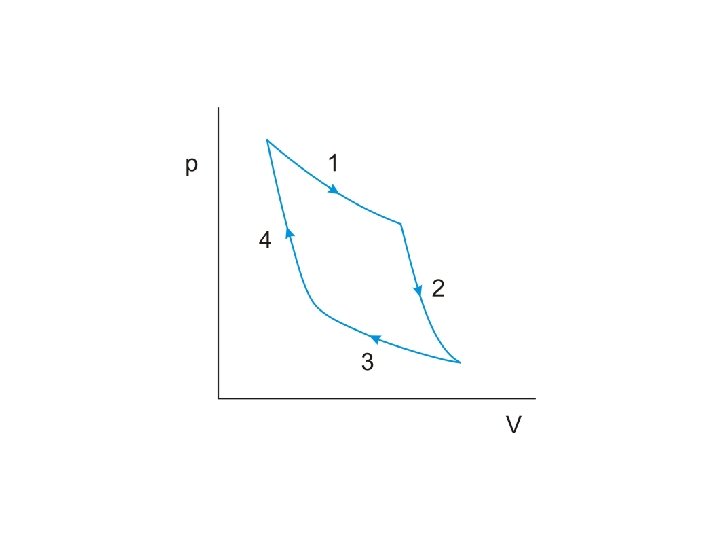
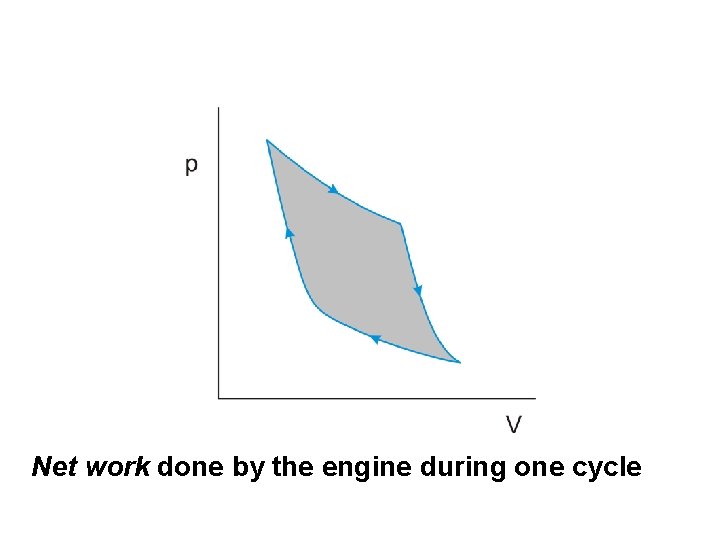
Net work done by the engine during one cycle

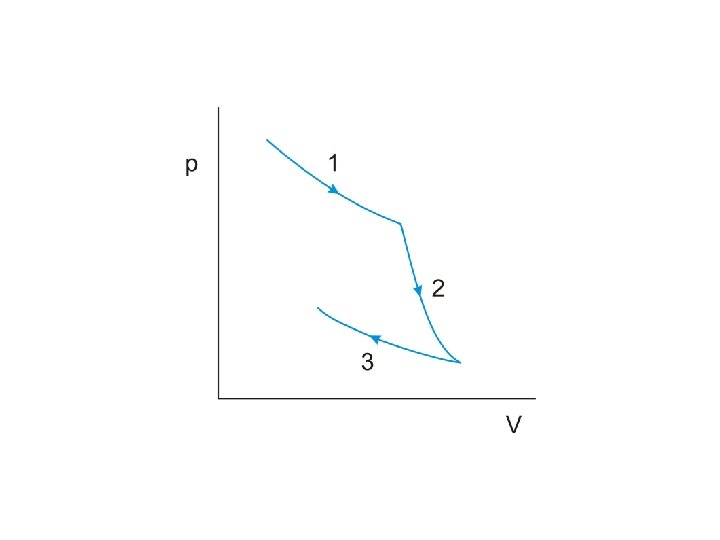
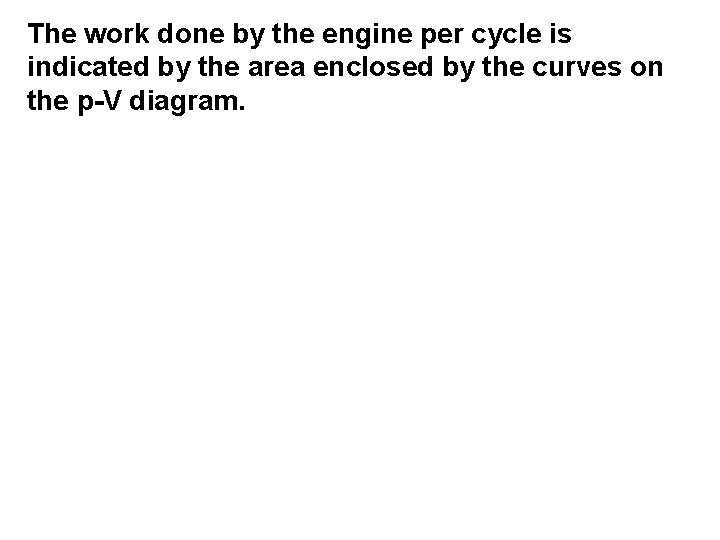
The work done by the engine per cycle is indicated by the area enclosed by the curves on the p-V diagram.

The work done by the engine per cycle is indicated by the area enclosed by the curves on the p-V diagram. An obvious way to increase this area is to

The work done by the engine per cycle is indicated by the area enclosed by the curves on the p-V diagram. An obvious way to increase this area is to increase the difference between the temperatures at which the two isothermal changes occur

The work done by the engine per cycle is indicated by the area enclosed by the curves on the p-V diagram. An obvious way to increase this area is to increase the difference between the temperatures at which the two isothermal changes occur We can therefore conclude that the efficiency of a heat engine will be improved if

The work done by the engine per cycle is indicated by the area enclosed by the curves on the p-V diagram. An obvious way to increase this area is to increase the difference between the temperatures at which the two isothermal changes occur We can therefore conclude that the efficiency of a heat engine will be improved if the difference between the temperatures of the heat source and heat sink is increased

It is important to notice that the Carnot cycle (as described above) is completely

It is important to notice that the Carnot cycle (as described above) is completely reversible

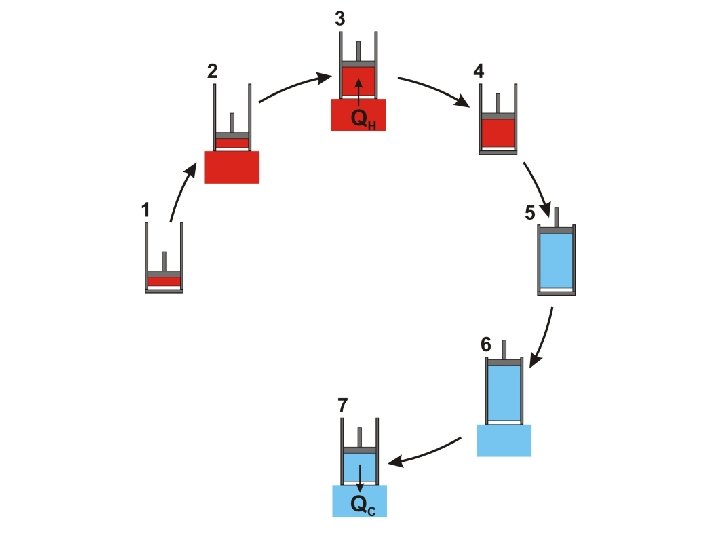
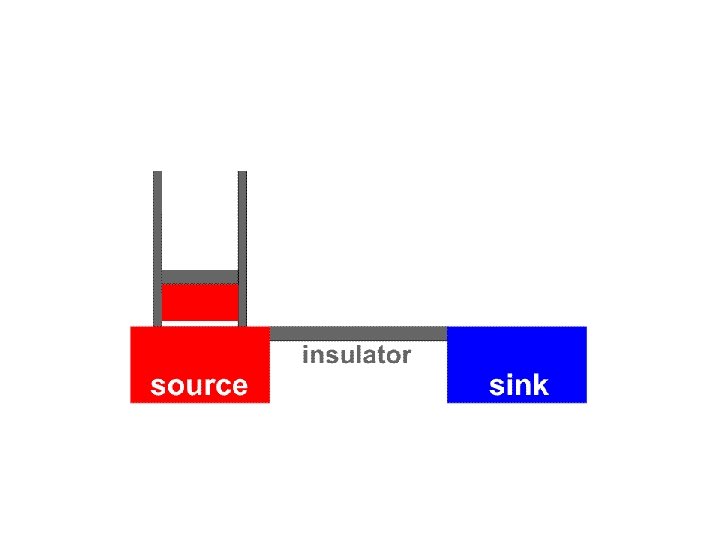
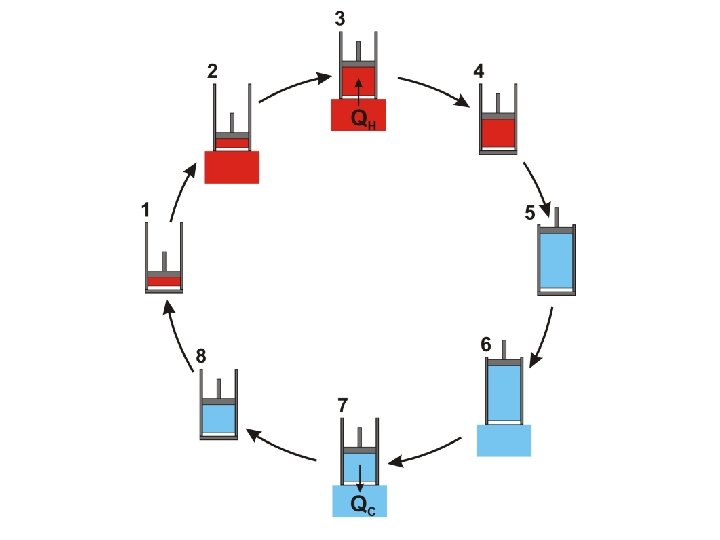
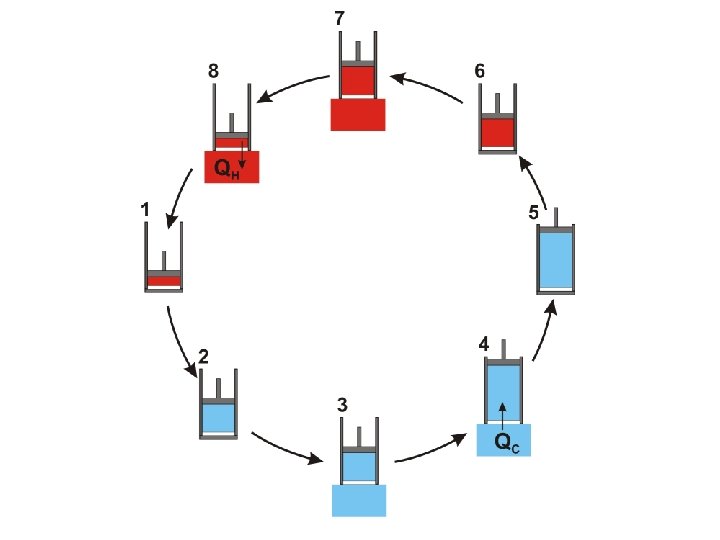
It is important to notice that the Carnot cycle (as described above) is completely reversible During the Carnot cycle, a quantity of energy is transferred from a hot body to a cold body and work is done by the gas



It is important to notice that the Carnot cycle (as described above) is completely reversible During the Carnot cycle, a quantity of energy is transferred from a hot body to a cold body and work is done by the gas If the procedure is reversed the same quantity of energy is transferred from a cold body to a hot body and the same quantity of work is done on the gas

Carnot suggested that for a given temperature difference between source and sink, no heat engine can be more efficient than a reversible engine

Carnot suggested that for a given temperature difference between source and sink, no heat engine can be more efficient than a reversible engine In practice, for the process to be (almost) reversible:

Carnot suggested that for a given temperature difference between source and sink, no heat engine can be more efficient than a reversible engine In practice, for the process to be (almost) reversible: The isothermal changes must be made

Carnot suggested that for a given temperature difference between source and sink, no heat engine can be more efficient than a reversible engine In practice, for the process to be (almost) reversible: The isothermal changes must be made very slowly

Carnot suggested that for a given temperature difference between source and sink, no heat engine can be more efficient than a reversible engine In practice, for the process to be (almost) reversible: The isothermal changes must be made very slowly The cylinder must be perfectly

Carnot suggested that for a given temperature difference between source and sink, no heat engine can be more efficient than a reversible engine In practice, for the process to be (almost) reversible: The isothermal changes must be made very slowly The cylinder must be perfectly insulated

Carnot suggested that for a given temperature difference between source and sink, no heat engine can be more efficient than a reversible engine In practice, for the process to be (almost) reversible: The isothermal changes must be made very slowly The cylinder must be perfectly insulated There must be no

Carnot suggested that for a given temperature difference between source and sink, no heat engine can be more efficient than a reversible engine In practice, for the process to be (almost) reversible: The isothermal changes must be made very slowly The cylinder must be perfectly insulated There must be no friction