The capture of CO 2 from flue gas






- Slides: 21

The capture of CO 2 from flue gas using adsorption combined with membrane separation Prof. Krzysztof Warmuzinski Polish Academy of Sciences Institute of Chemical Engineering 6 th International Scientific Conference on Energy and Climate Change

CO 2 CAPTURE v Post-combustion systems v Pre-combustion systems v Oxyfuel combustion Post-combustion systems, which separate CO 2 from flue gases produced by the combustion of a primary fossil fuel (coal, natural gas, oil) or biomass in air 6 th International Scientific Conference on Energy and Climate Change

POST-COMBUSTION CO 2 CAPTURE v v Absorption Membrane separation Adsorption (PSA, TSA) Hybrid systems 6 th International Scientific Conference on Energy and Climate Change

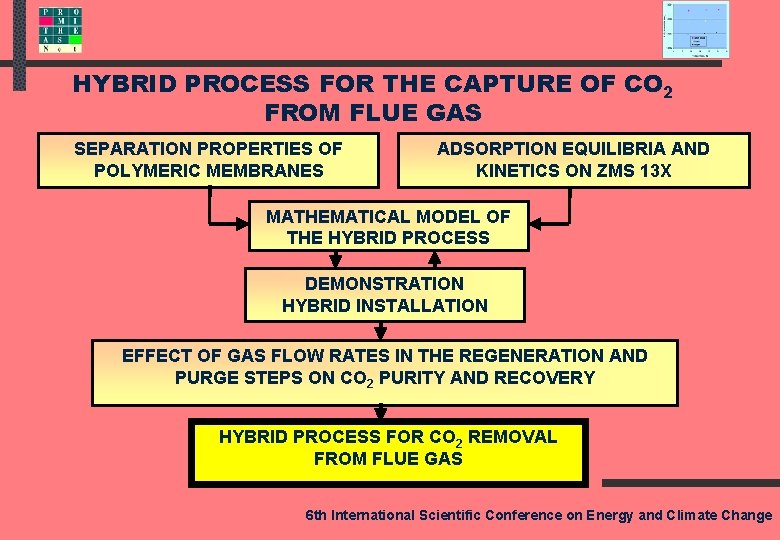
HYBRID PROCESS FOR THE CAPTURE OF CO 2 FROM FLUE GAS SEPARATION PROPERTIES OF POLYMERIC MEMBRANES ADSORPTION EQUILIBRIA AND KINETICS ON ZMS 13 X MATHEMATICAL MODEL OF THE HYBRID PROCESS DEMONSTRATION HYBRID INSTALLATION EFFECT OF GAS FLOW RATES IN THE REGENERATION AND PURGE STEPS ON CO 2 PURITY AND RECOVERY HYBRID PROCESS FOR CO 2 REMOVAL FROM FLUE GAS 6 th International Scientific Conference on Energy and Climate Change

Demonstration hybrid installation 6 th International Scientific Conference on Energy and Climate Change

Demonstration hybrid installation 6 th International Scientific Conference on Energy and Climate Change

Objectives of the study v v Theoretical and experimental determination of the principal parameters of product streams (i. e. the purified gas stream and the CO 2 -rich stream), especially from the standpoint of purity requirements associated with the transport and storage of CO 2 Analysis of the effect of two crucial process parameters – gas flow rates in the regeneration and purge steps – on the recovery of CO 2 and its concentration in the enriched stream 6 th International Scientific Conference on Energy and Climate Change

The PSA cycle 6 th International Scientific Conference on Energy and Climate Change

Basic parameters of the process 6 th International Scientific Conference on Energy and Climate Change

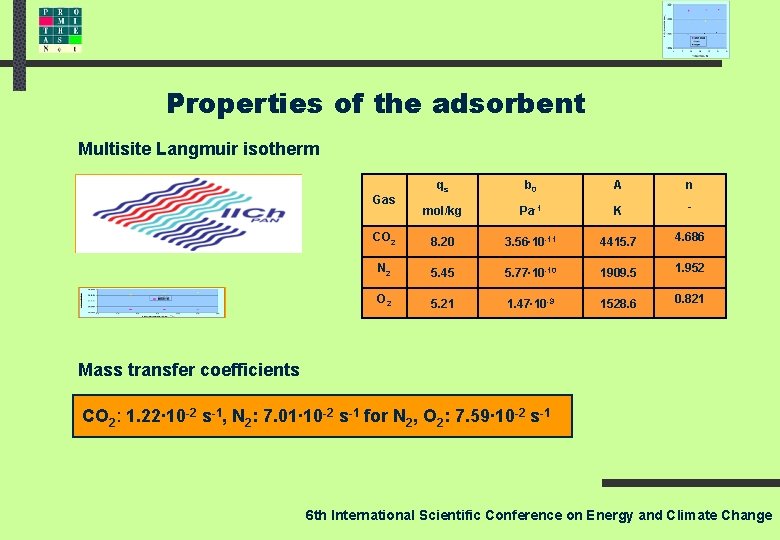
Properties of the adsorbent 6 th International Scientific Conference on Energy and Climate Change

Properties of the adsorbent Multisite Langmuir isotherm qs b 0 A n mol/kg Pa-1 K - CO 2 8. 20 3. 56∙ 10 -11 4415. 7 4. 686 N 2 5. 45 5. 77∙ 10 -10 1909. 5 1. 952 O 2 5. 21 1. 47∙ 10 -9 1528. 6 0. 821 Gas Mass transfer coefficients CO 2: 1. 22∙ 10 -2 s-1, N 2: 7. 01∙ 10 -2 s-1 for N 2, O 2: 7. 59∙ 10 -2 s-1 6 th International Scientific Conference on Energy and Climate Change

Properties of the membrane module 6 th International Scientific Conference on Energy and Climate Change

Modelling of the hybrid separation of CO 2 from flue gas streams v v v Model of the PSA separation Model of the membrane separation Integration of the PSA and membrane models into PSE g. PROMS software package 6 th International Scientific Conference on Energy and Climate Change

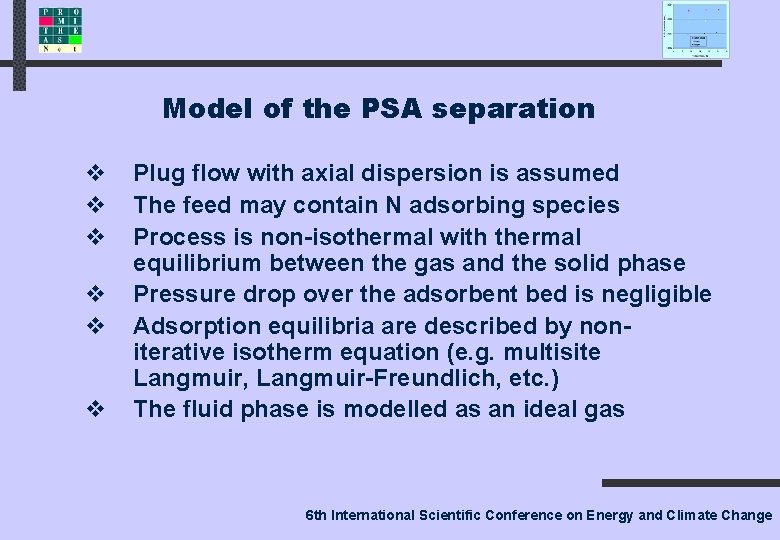
Model of the PSA separation v v v Plug flow with axial dispersion is assumed The feed may contain N adsorbing species Process is non-isothermal with thermal equilibrium between the gas and the solid phase Pressure drop over the adsorbent bed is negligible Adsorption equilibria are described by noniterative isotherm equation (e. g. multisite Langmuir, Langmuir-Freundlich, etc. ) The fluid phase is modelled as an ideal gas 6 th International Scientific Conference on Energy and Climate Change

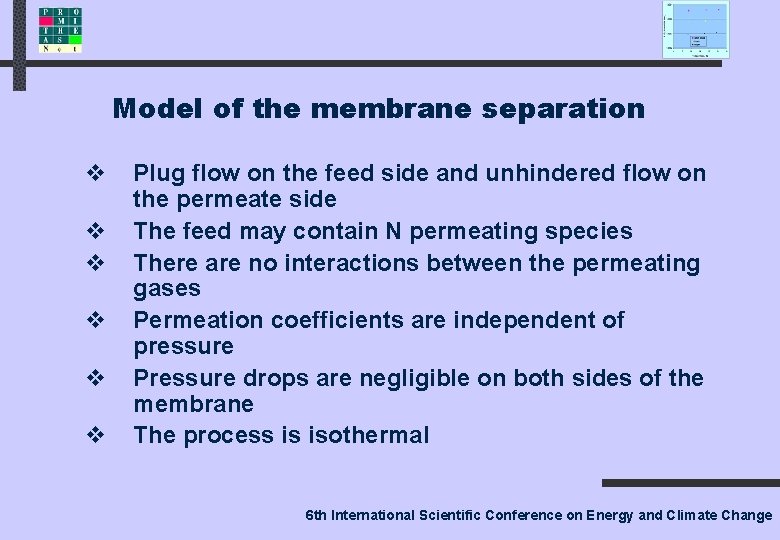
Model of the membrane separation v v v Plug flow on the feed side and unhindered flow on the permeate side The feed may contain N permeating species There are no interactions between the permeating gases Permeation coefficients are independent of pressure Pressure drops are negligible on both sides of the membrane The process is isothermal 6 th International Scientific Conference on Energy and Climate Change

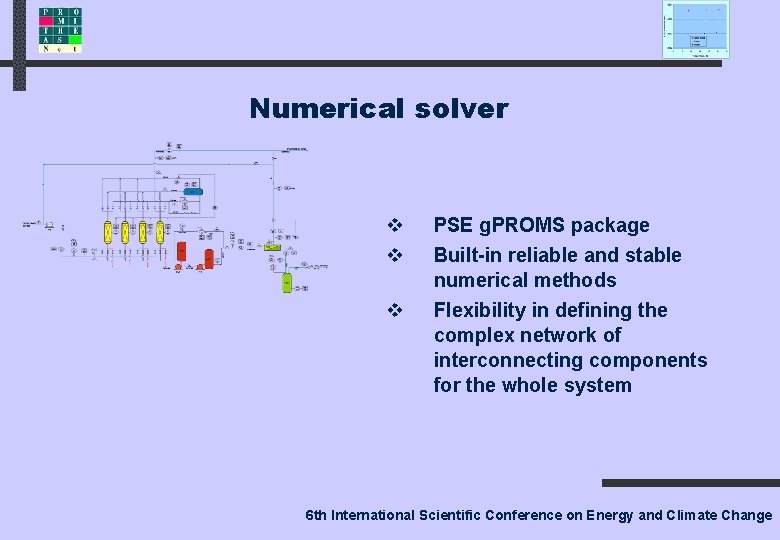
Numerical solver v v v PSE g. PROMS package Built-in reliable and stable numerical methods Flexibility in defining the complex network of interconnecting components for the whole system 6 th International Scientific Conference on Energy and Climate Change

CO 2 recovery and its concentration in the enriched gas Gas flow rates in the purge step of the PSA cycle: 6. 4 m 3(STP)/h 6. 8 m 3(STP)/h 7. 2 m 3(STP)/h 6 th International Scientific Conference on Energy and Climate Change

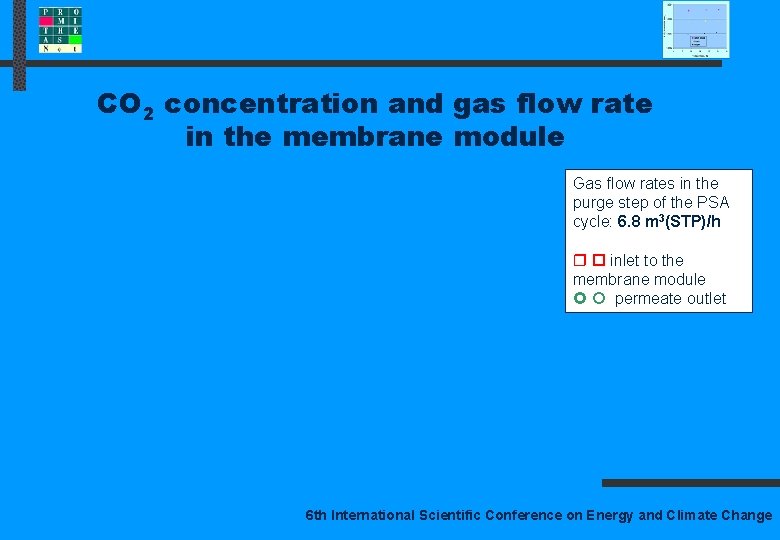
CO 2 concentration and gas flow rate in the membrane module Gas flow rates in the purge step of the PSA cycle: 6. 8 m 3(STP)/h inlet to the membrane module permeate outlet 6 th International Scientific Conference on Energy and Climate Change

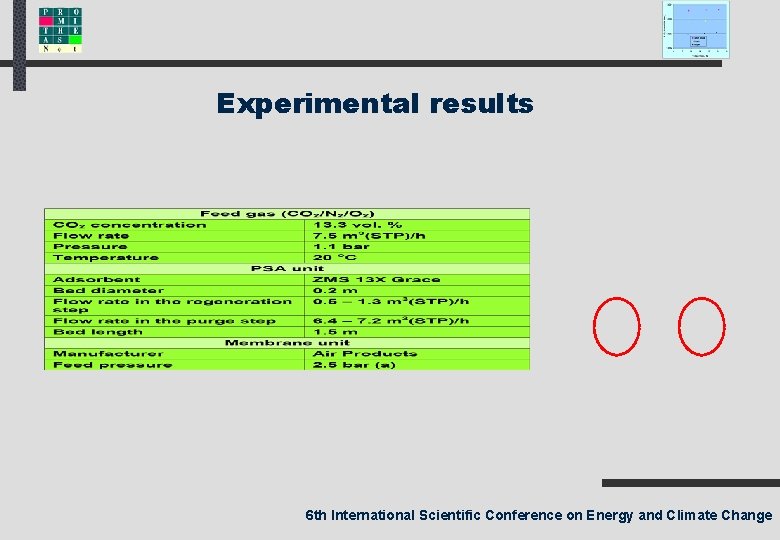
Experimental results 6 th International Scientific Conference on Energy and Climate Change

CONCLUSIONS v v v In the process analyzed, it is possible to raise CO 2 concentration from 13. 3 vol. % to over 97 vol. %, with a virtually total recovery The CO 2 concentrations obtained are sufficient from the standpoint of CO 2 transportation and storage For three different flow rates during purge with the enriched stream (6. 4, 6. 8 and 7. 2 m 3 (STP)/h) the limiting values were determined for the regenerating streams (0. 7, 0. 9 and 1. 1 m 3 (STP)/h, respectively). These values lead to the maximum CO 2 concentrations in the enriched product without any CO 2 breakthrough into the purified stream 6 th International Scientific Conference on Energy and Climate Change

THANK YOU FOR YOUR ATTENTION 6 th International Scientific Conference on Energy and Climate Change