The canine model in the cancer fight role













- Slides: 23

The canine model in the cancer fight: role of comparative oncology in translational research “Cancer translational research: from molecular biology to the real world setting” Sala Convegni – Ce. S. I. – Me. T 20 April 2018, Chieti

Comparative Oncology discipline that integrates the study of naturally occurring cancers in veterinary patients into studies of human cancer biology and therapy The biological complexity of cancer in pet animals captures the essence of cancer in human patients

Advantages of naturally occurring cancers for translational research: Ø naturally-occurring, with individual-to-individual heterogeneity within and across cancer types Ø growth in immunocompetent organisms within the tumor microenvironment Ø shorter natural history Ø sharing of the same environment with humans Ø highly comparable risk factors

Advantages of naturally occurring cancers for translational research: • Spontaneous cancers in pets share tumor biology /genetics and clinical behaviour with human cancers • Similar tumor histology and response rates to conventional chemotherapy • Sufficient prevalence for biological studies and clinical trials • Feasibility of multi-modality protocols • Rapid progression and early metastatic failure rapid completion of clinical trials

Main objectives of comparative oncology: - Study of cancer pathogenesis cancer-associated genes and proteins - Understanding of environmental risk factors for cancer - Examination of genetic/familial determinants for cancer predispositions - Development of new treatment options for the management of cancer in both humans and animals

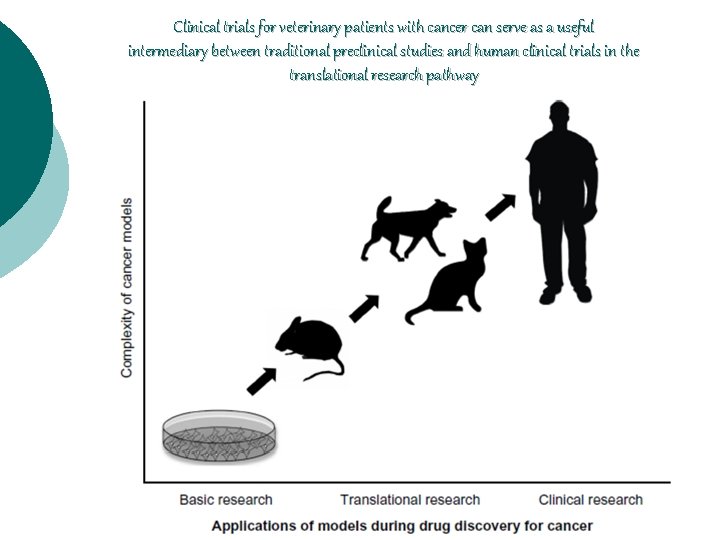
Clinical trials for veterinary patients with cancer can serve as a useful intermediary between traditional preclinical studies and human clinical trials in the translational research pathway

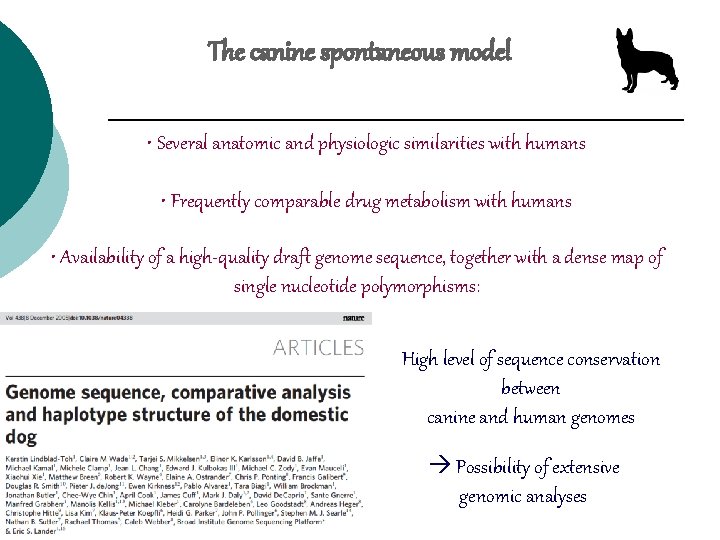
The canine spontaneous model • Several anatomic and physiologic similarities with humans • Frequently comparable drug metabolism with humans • Availability of a high-quality draft genome sequence, together with a dense map of single nucleotide polymorphisms: High level of sequence conservation between canine and human genomes Possibility of extensive genomic analyses

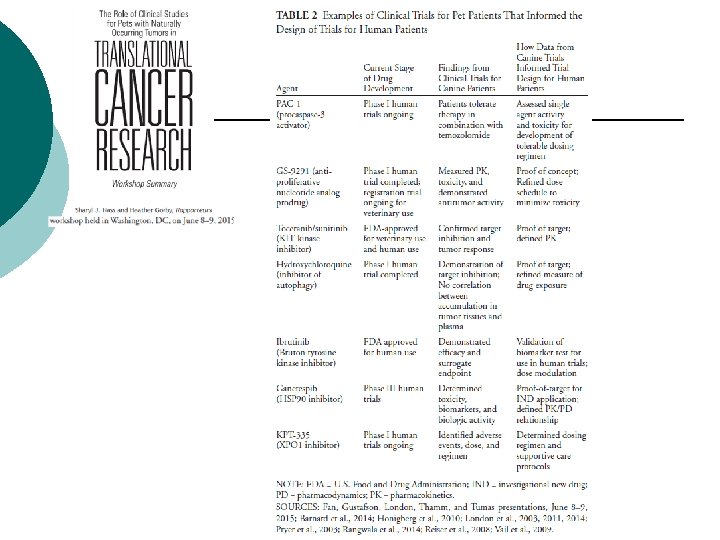
Advantages of Clinical Trials for Canine Patients in Translational Cancer Research • Use data from clinical trials for canine patients to inform the design of subsequent clinical trials for human patients (to select the best compound and to prioritize the most promising combinations) • Learn from trials enrolling dogs with treatment-naïve disease (many human patients enrolled in trials have advanced, treatment-resistant disease) • Develop trials for frequently occurring cancers in dogs in order to address unmet needs for rare human cancers • Use genomic information from canine patients to identify cancer genes not yet identified in humans and to accelerate the development of targeted therapies

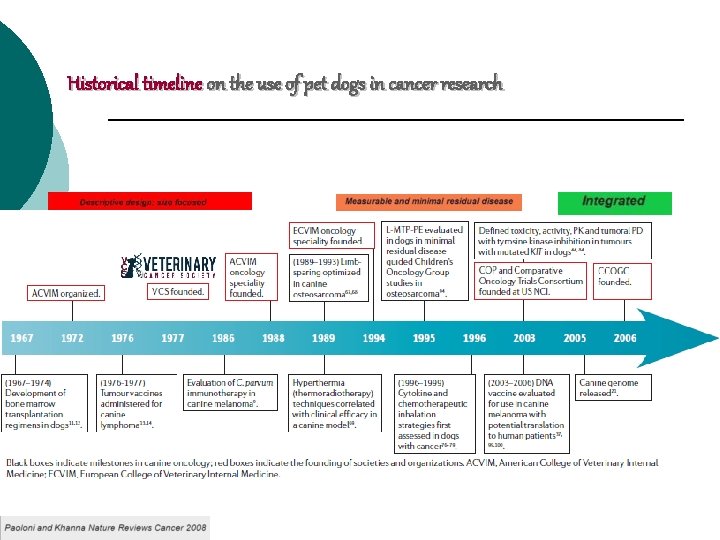
Historical timeline on the use of pet dogs in cancer research

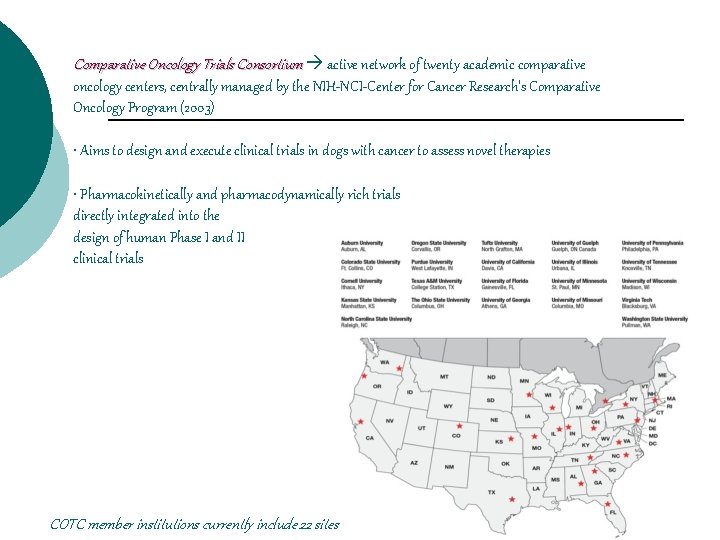
Comparative Oncology Trials Consortium active network of twenty academic comparative oncology centers, centrally managed by the NIH-NCI-Center for Cancer Research's Comparative Oncology Program (2003) • Aims to design and execute clinical trials in dogs with cancer to assess novel therapies • Pharmacokinetically and pharmacodynamically rich trials directly integrated into the design of human Phase I and II clinical trials COTC member institutions currently include 22 sites

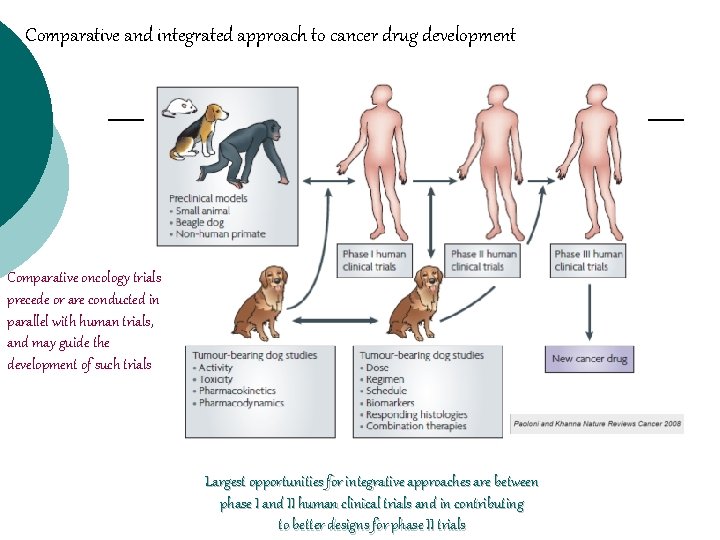
Comparative and integrated approach to cancer drug development Comparative oncology trials precede or are conducted in parallel with human trials, and may guide the development of such trials Largest opportunities for integrative approaches are between phase I and II human clinical trials and in contributing to better designs for phase II trials


https: //ccr. cancer. gov/comparative-oncology-program The use of the dog as a model of cancer drug translation has not been exploited as widely in Europe as in the United States Clinical trials for pet patients with naturally occurring tumors are underutilized in the drug development process

Mainly studied cancer types in translational research Lymphoma • Among the most common types of tumors in dogs • Remarkable similarities between clinical features and cytogenetic aberrations of canine lymphomas and human NHL • More than 50 years ago, optimization of bone marrow transplantation protocols was undertaken in dogs (Thomas et al. , 1962; Epstein et al. , 1969)

Sarcomas • Sarcomas are more prevalent in dogs than in humans • Significant similarities between genomic profiles in dog and human soft tissue sarcomas

Osteosarcoma • Strong similarity in the global expression patterns of canine and human OSA • Limb-sparing techniques optimized in canine OSA (La. Rue et al. , 1989; Withrow et al. , 1993)

Brain tumours - Glioma

Melanoma Urinary bladder cancer

Similar if not identical cancer-causing gene mutations can also result in different cancers in humans and dogs: Mast cell tumours (MCT) • Similar mutations in KIT identified in both GIST in humans and MCT in dogs • Studies in dogs with MCT aided translational development of tyrosine kinase inhibitors by defining toxicity, activity, pharmacokinetic and tumour pharmacodynamic relationships • High prevalence of canine MCT provides significant opportunities to study therapeutic strategies targeting KIT-driven cancers

Canine spontaneous tumours valuable but underutilized model Perceived concerns • Study duration timelines for completion of studies in pet dogs are longer than those in rodent models • Cancer prevalence • Drug and budget requirements • Patient-to-patient variability concern that the ‘uncontrolled’ nature of pet dog studies may falsely associate a toxicity with a new cancer drug • Species concerns canine gastrointestinal sensitivity recognized to be higher than human patients

Cancer research community has not reached agreement concerning: - the value of clinical trial data in dogs for advancing human cancer research - when and how best to integrate comparative oncology trials within the cancer research continuum

Future needs and directions - Availability of large, highly trained, multi-disciplinary teams of investigators to design studies that are integrated within the development path of cancer drugs - Conduction of studies under clear regulatory guidance ( standardized guidelines) and results translated to studies in human patients

“Between animal and human medicine there is no dividing line—nor should there be. The object is different but the experience obtained constitutes the basis of all medicine. ” — Rudolf Virchow (1821– 1902) Conquering cancer: Walking the path together (Le. Blanc et al. , 2015) Thank you for your attention!