The Breast Course Breast Cancer Biologicals New Paradigms

The Breast Course Breast Cancer: Biologicals New Paradigms ----------------Joseph Ragaz, Director, Oncology Program Mc. Gill University Hospital Center May 5 2006
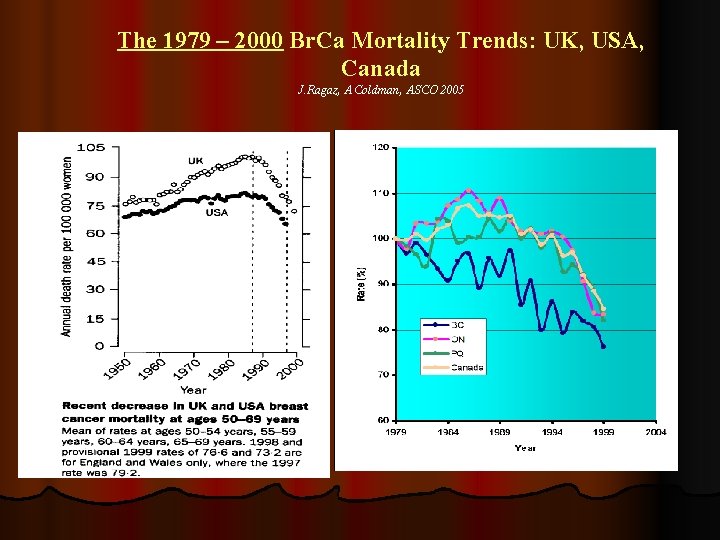
The 1979 – 2000 Br. Ca Mortality Trends: UK, USA, Canada J. Ragaz, A. Coldman, ASCO 2005

Breast Cancer: Mortality Reduction l Education & screening & downstaging l Endocrine (Tamoxifen & AIs) l Chemotherapy l Radiotherapy l. Can we cure more pts? l. Molecular Biology

Breast Cancer: Targeted Therapy l Tumor Biology l Biologicals
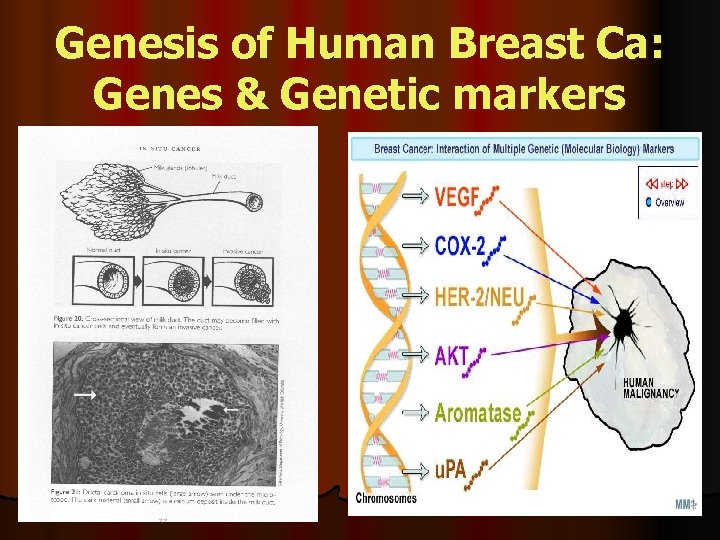
Genesis of Human Breast Ca: Genes & Genetic markers
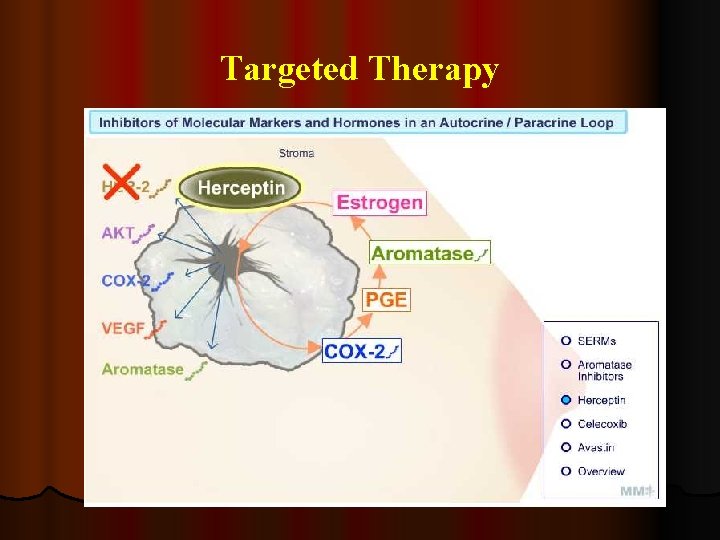
Targeted Therapy

Her 2/Neu
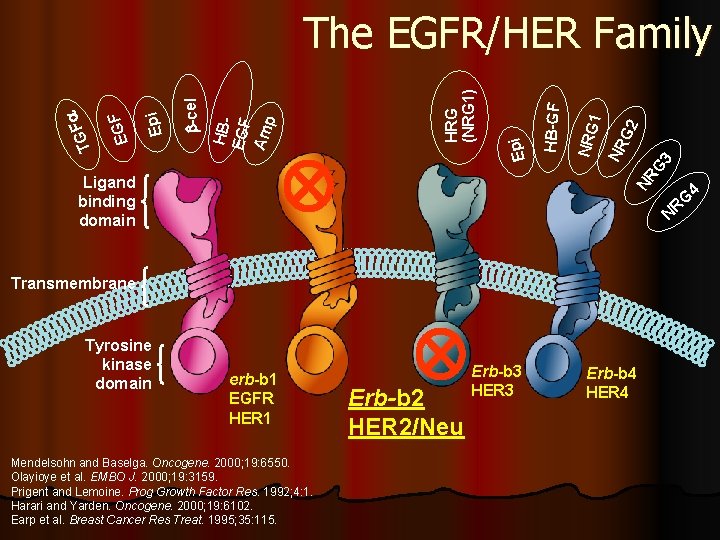
G 2 G 3 NR 1 NRG NR Ligand binding domain HB-GF Epi HRG (NRG 1) HBEGF Am p -cel Epi EGF TG F The EGFR/HER Family N Transmembrane Tyrosine kinase domain 4 RG erb-b 1 EGFR HER 1 Mendelsohn and Baselga. Oncogene. 2000; 19: 6550. Olayioye et al. EMBO J. 2000; 19: 3159. Prigent and Lemoine. Prog Growth Factor Res. 1992; 4: 1. Harari and Yarden. Oncogene. 2000; 19: 6102. Earp et al. Breast Cancer Res Treat. 1995; 35: 115. Erb-b 2 HER 2/Neu Erb-b 3 HER 3 Erb-b 4 HER 4
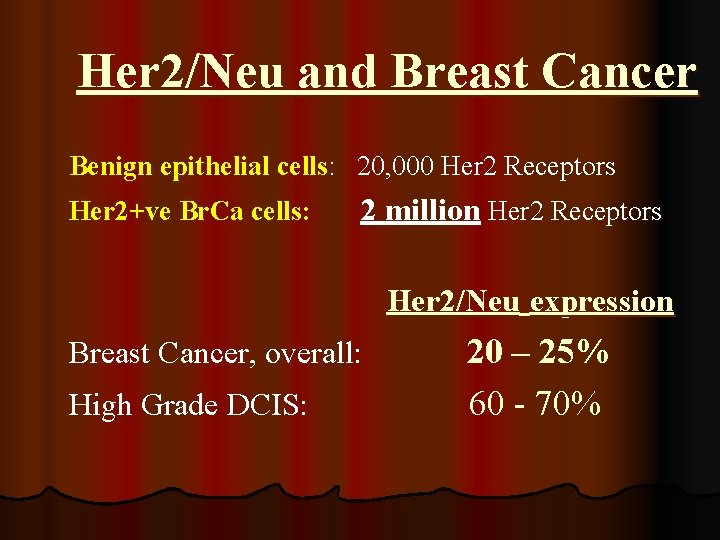
Her 2/Neu and Breast Cancer Benign epithelial cells: 20, 000 Her 2 Receptors Her 2+ve Br. Ca cells: 2 million Her 2 Receptors Her 2/Neu expression Breast Cancer, overall: High Grade DCIS: 20 – 25% 60 - 70%
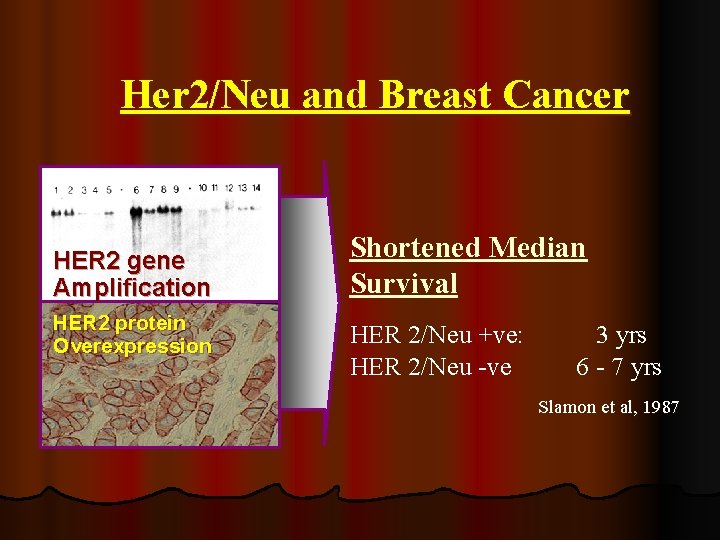
Her 2/Neu and Breast Cancer HER 2 gene Amplification HER 2 protein Overexpression Shortened Median Survival HER 2/Neu +ve: HER 2/Neu -ve 3 yrs 6 - 7 yrs Slamon et al, 1987
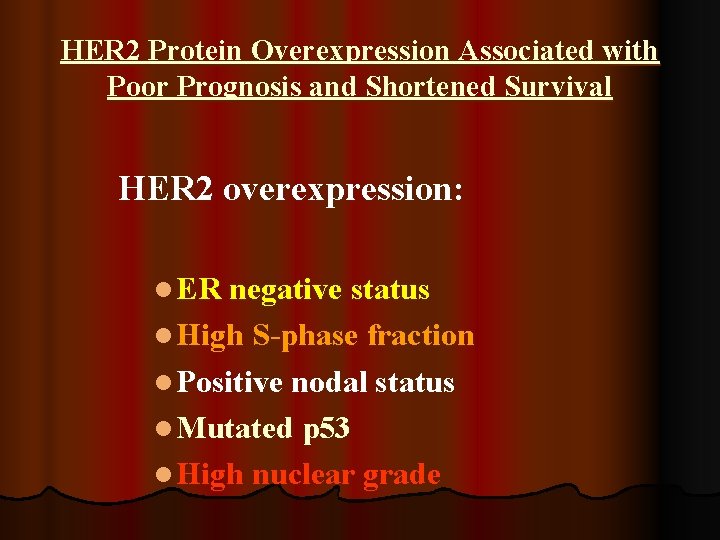
HER 2 Protein Overexpression Associated with Poor Prognosis and Shortened Survival HER 2 overexpression: l ER negative status l High S-phase fraction l Positive nodal status l Mutated p 53 l High nuclear grade
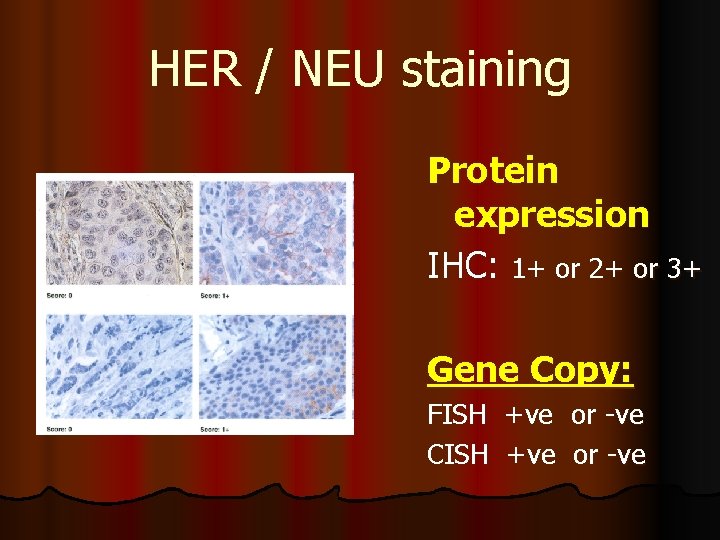
HER / NEU staining Protein expression IHC: 1+ or 2+ or 3+ Gene Copy: FISH +ve or -ve CISH +ve or -ve

Herceptin: Humanized Anti-HER 2 Antibody l Targets HER 2 oncoprotein l High affinity (Kd = 0. 1 n. M) and specificity l 95% human, 5% murine l Decrease potential for immunogenicity l Increase potential for recruiting immune-effector mechanisms
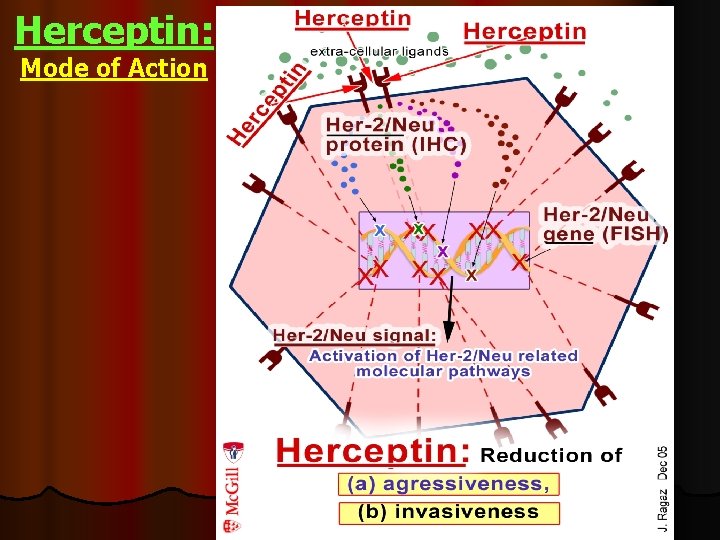
Herceptin: Mode of Action
Herceptin and Chemotherapy l. Additive effect l. Synergistic effect
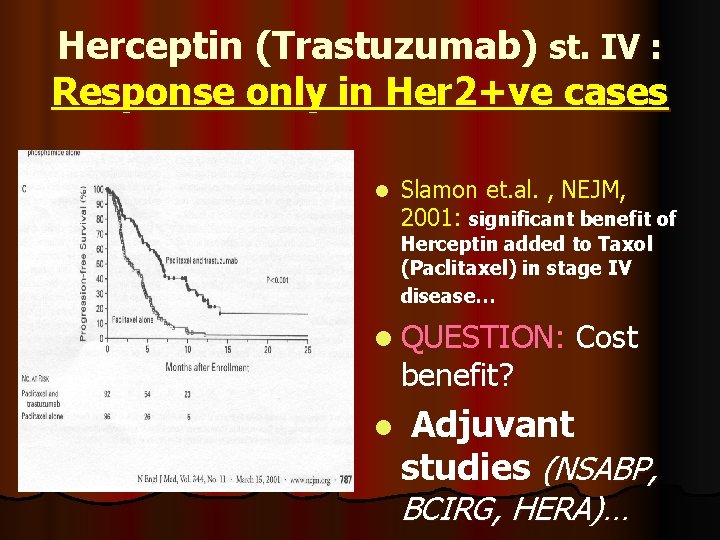
Herceptin (Trastuzumab) st. IV : Response only in Her 2+ve cases l Slamon et. al. , NEJM, 2001: significant benefit of Herceptin added to Taxol (Paclitaxel) in stage IV disease… l QUESTION: benefit? l Cost Adjuvant studies (NSABP, BCIRG, HERA)…
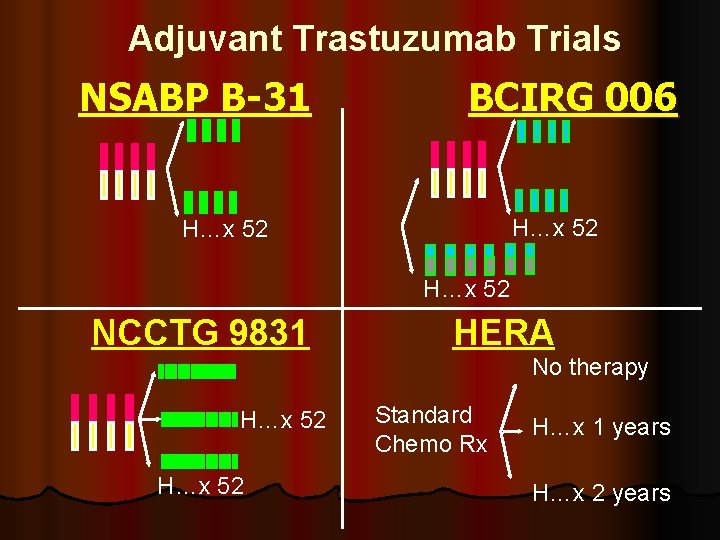
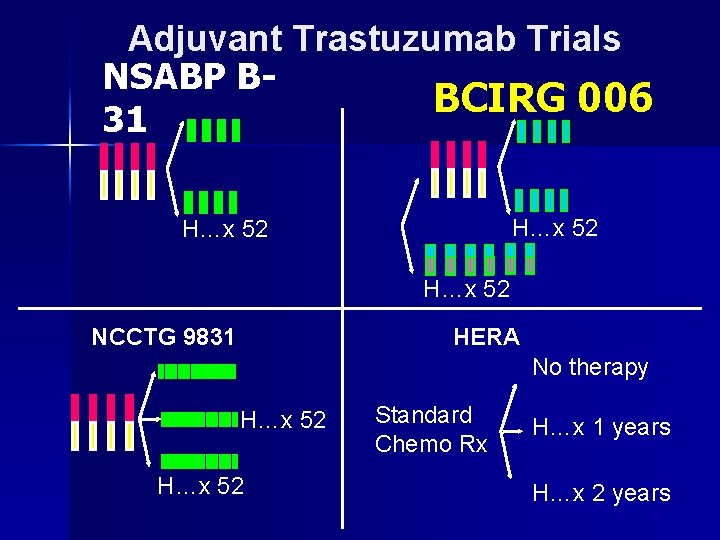
Adjuvant Trastuzumab Trials NSABP B-31 BCIRG 006 H…x 52 NCCTG 9831 HERA No therapy H…x 52 Standard Chemo Rx H…x 1 years H…x 2 years
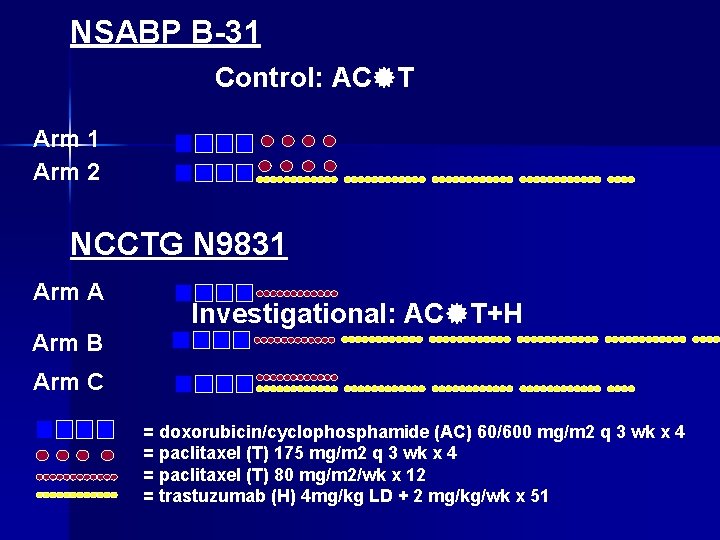
NSABP B-31 Control: AC T Arm 1 Arm 2 NCCTG N 9831 Arm A Investigational: AC T+H Arm B Arm C = doxorubicin/cyclophosphamide (AC) 60/600 mg/m 2 q 3 wk x 4 = paclitaxel (T) 175 mg/m 2 q 3 wk x 4 = paclitaxel (T) 80 mg/m 2/wk x 12 = trastuzumab (H) 4 mg/kg LD + 2 mg/kg/wk x 51

NSABP B-31: Herceptin trial. Her 2/Neu +ve cases ARM 1: AC – Taxol n ARM 2: AC – Taxol + Herceptin x 1 year n
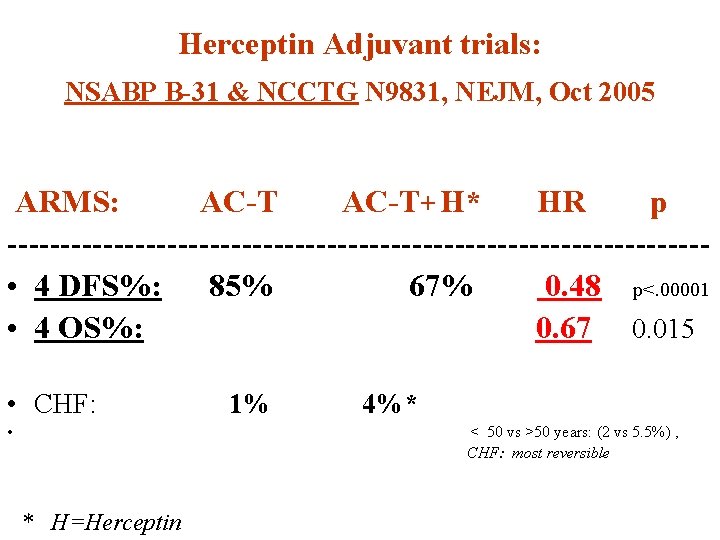
Herceptin Adjuvant trials: NSABP B-31 & NCCTG N 9831, NEJM, Oct 2005 ARMS: AC-T+ H* HR p --------------------------------- • 4 DFS%: 85% 67% 0. 48 p<. 00001 • 4 OS%: 0. 67 0. 015 • CHF: • 1% 4%* < 50 vs >50 years: (2 vs 5. 5%) , CHF: most reversible * H=Herceptin
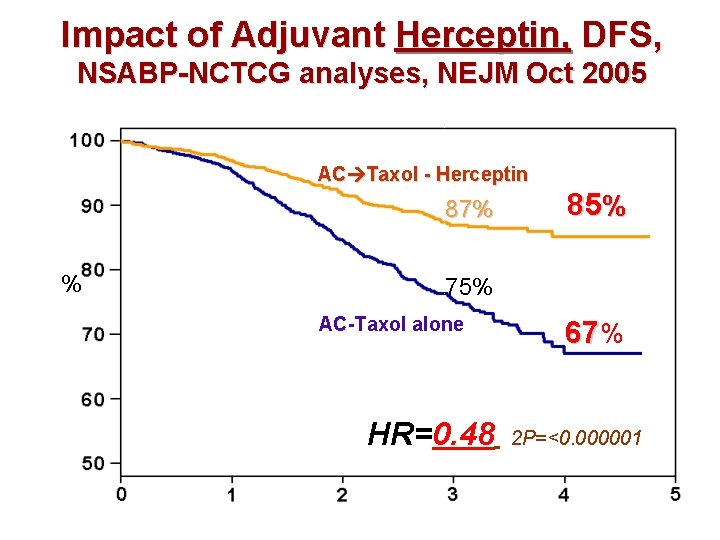
Impact of Adjuvant Herceptin, DFS, NSABP-NCTCG analyses, NEJM Oct 2005 AC Taxol - Herceptin AC T % 85% 87% 75% AC-Taxol alone HR=0. 48 67% 2 P=<0. 000001 Years From Randomization

Targeting “Targeted Therapy” • Can we identify subsets who will benefit (from Herceptin) much more, and those who will benefit much less…
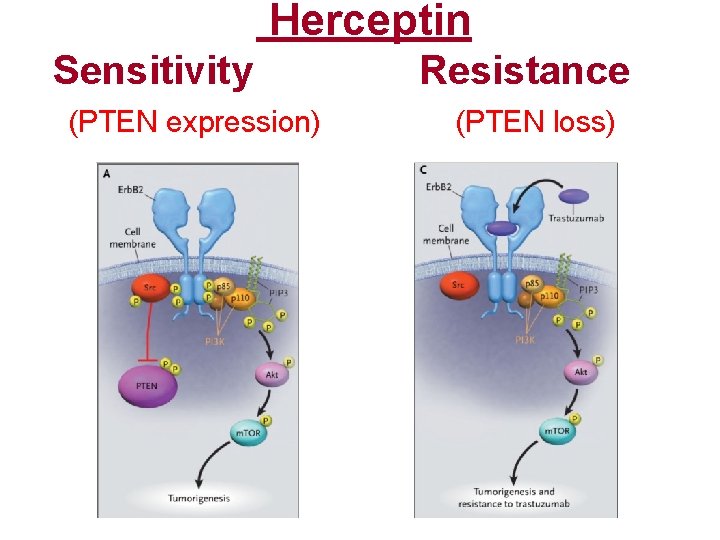
Herceptin Sensitivity (PTEN expression) Resistance (PTEN loss)
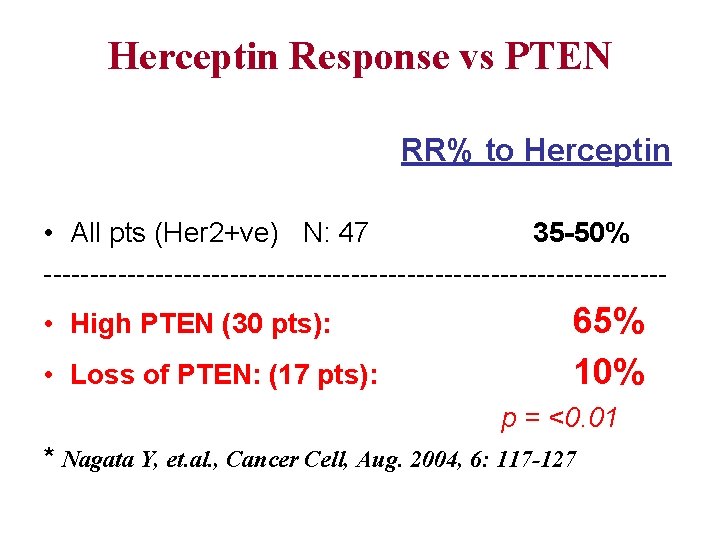
Herceptin Response vs PTEN RR% to Herceptin • All pts (Her 2+ve) N: 47 35 -50% --------------------------------- • High PTEN (30 pts): • Loss of PTEN: (17 pts): 65% 10% p = <0. 01 * Nagata Y, et. al. , Cancer Cell, Aug. 2004, 6: 117 -127

• Role of c-Myc
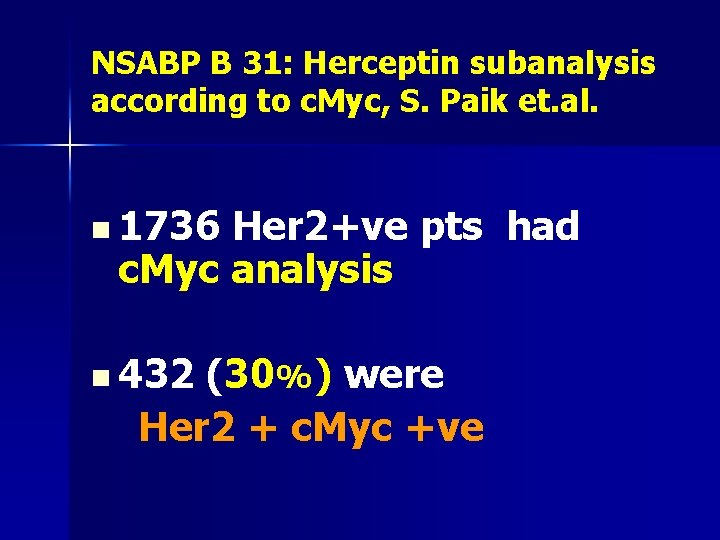
NSABP B 31: Herceptin subanalysis according to c. Myc, S. Paik et. al. n 1736 Her 2+ve pts had c. Myc analysis n 432 (30%) were Her 2 + c. Myc +ve
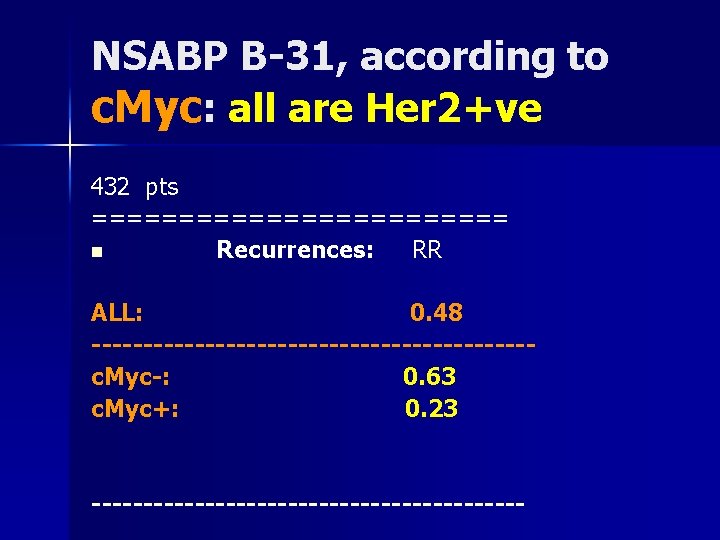
NSABP B-31, according to c. Myc: all are Her 2+ve 432 pts ============ n Recurrences: RR ALL: 0. 48 ---------------------c. Myc-: 0. 63 c. Myc+: 0. 23 ---------------------
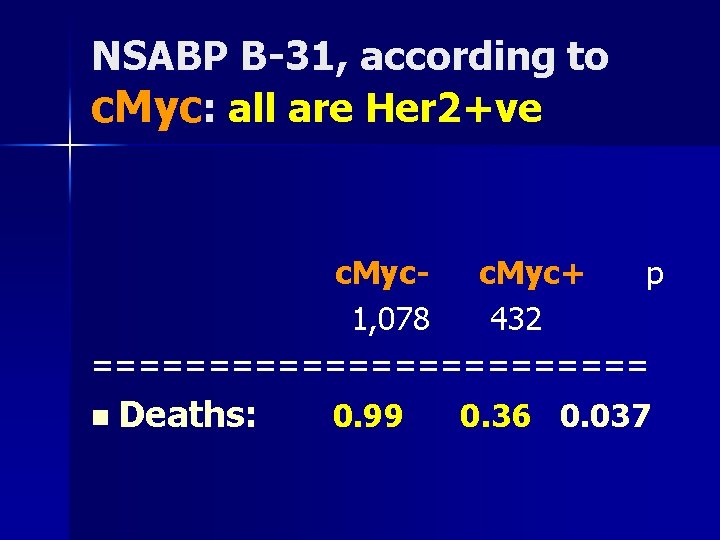
NSABP B-31, according to c. Myc: all are Her 2+ve c. Myc+ p 1, 078 432 ============ n Deaths: 0. 99 0. 36 0. 037

San Antonio 2005 n. DCIS and Her 2/Neu expression
Adjuvant Trastuzumab Trials NSABP BBCIRG 006 31 H…x 52 NCCTG 9831 HERA No therapy H…x 52 Standard Chemo Rx H…x 1 years H…x 2 years
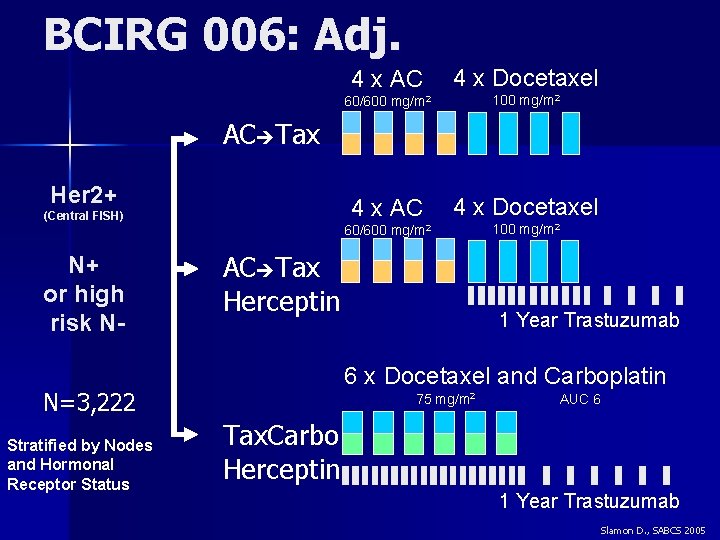
BCIRG 006: Adj. 4 x AC 4 x Docetaxel 60/600 mg/m 2 100 mg/m 2 AC Tax Her 2+ (Central FISH) N+ or high risk N- AC Tax Herceptin 6 x Docetaxel and Carboplatin N=3, 222 Stratified by Nodes and Hormonal Receptor Status 1 Year Trastuzumab 75 mg/m 2 AUC 6 Tax. Carbo Herceptin 1 Year Trastuzumab Slamon D. , SABCS 2005
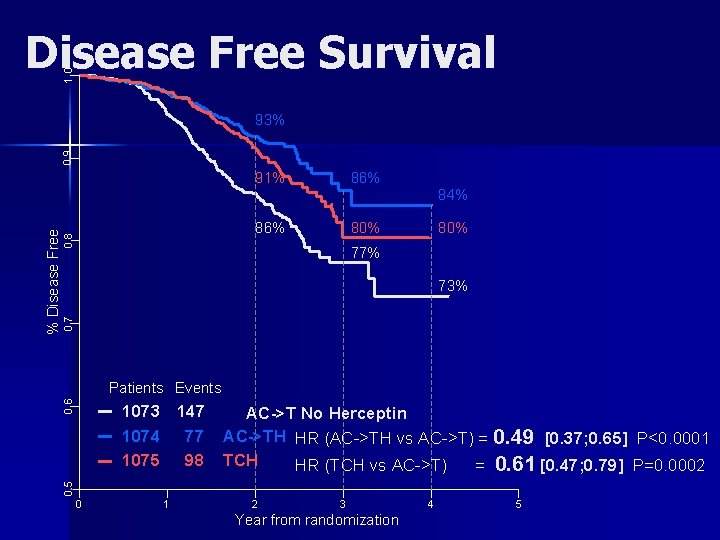
1. 0 Disease Free Survival 0. 8 91% 86% 80% 84% 80% 77% 0. 7 73% 0. 6 Patients Events 1073 147 1074 77 1075 98 AC->T No Herceptin AC->TH HR (AC->TH vs AC->T) = 0. 49 [0. 37; 0. 65] P<0. 0001 TCH HR (TCH vs AC->T) = 0. 61 [0. 47; 0. 79] P=0. 0002 0. 5 % Disease Free 0. 9 93% 0 1 2 3 Year from randomization 4 5

CARDIAC TOXICITY
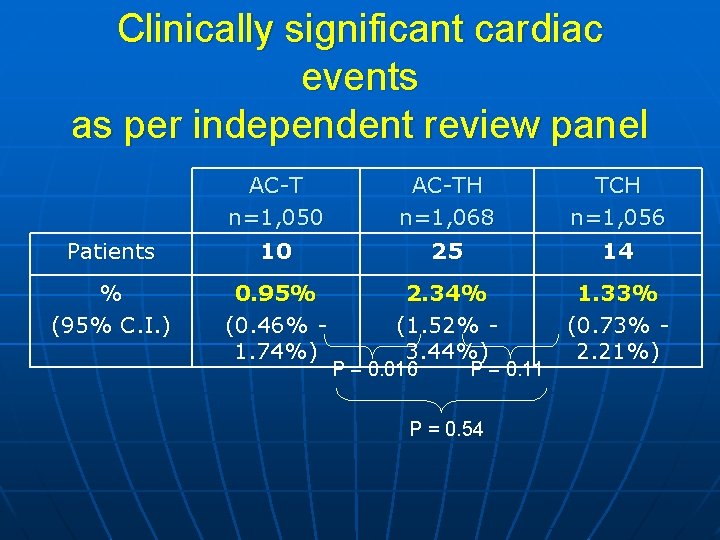
Clinically significant cardiac events as per independent review panel Patients % (95% C. I. ) AC-T n=1, 050 10 AC-TH n=1, 068 25 TCH n=1, 056 14 0. 95% (0. 46% 1. 74%) 2. 34% (1. 52% 3. 44%) 1. 33% (0. 73% 2. 21%) P = 0. 016 P = 0. 11 P = 0. 54
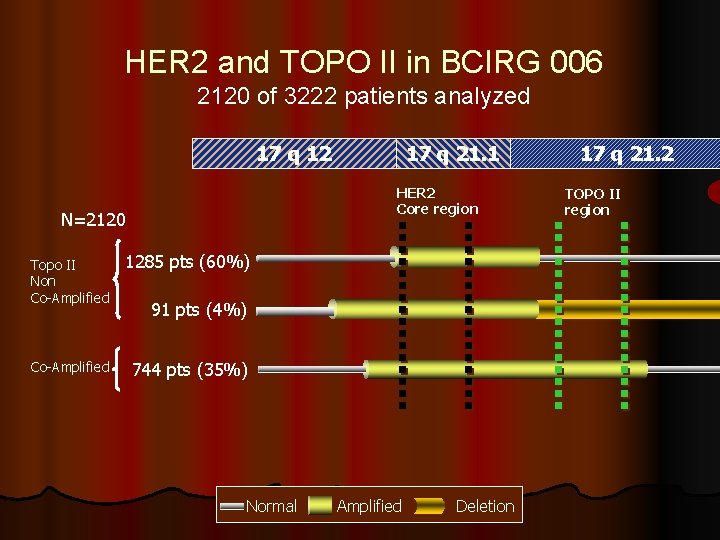
HER 2 and TOPO II in BCIRG 006 2120 of 3222 patients analyzed 17 q 12 17 q 21. 1 HER 2 Core region N=2120 Topo II Non Co-Amplified 1285 pts (60%) Co-Amplified 744 pts (35%) 91 pts (4%) Normal Amplified Deletion 17 q 21. 2 TOPO II region
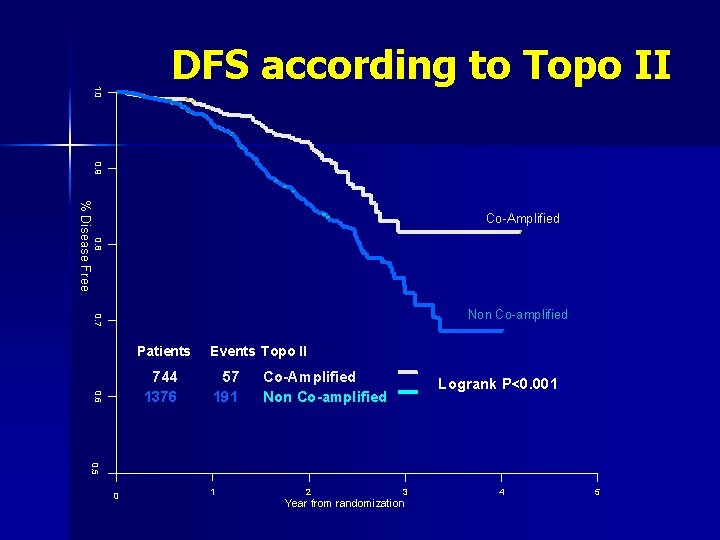
1. 0 DFS according to Topo II 0. 9 % Disease Free Co-Amplified 0. 8 0. 7 Non Co-amplified Patients 0. 6 744 1376 Events Topo II 57 191 Co-Amplified Non Co-amplified Logrank P<0. 001 0. 5 0 1 2 3 Year from randomization 4 5
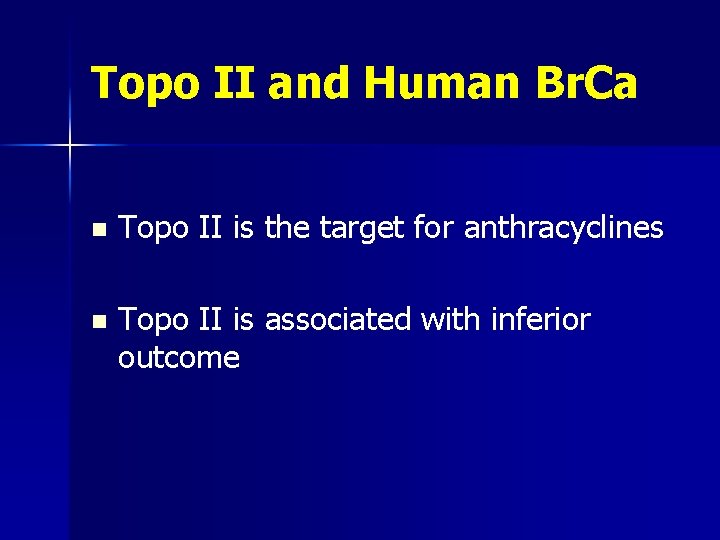
Topo II and Human Br. Ca n Topo II is the target for anthracyclines n Topo II is associated with inferior outcome
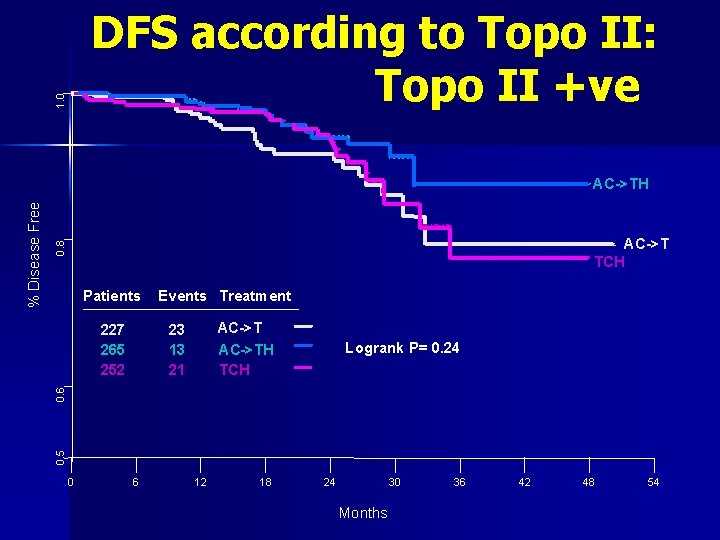
1. 0 DFS according to Topo II: Topo II +ve 0. 8 AC->T TCH Patients AC->TH TCH 23 13 21 Logrank P= 0. 24 0. 6 227 265 252 Events Treatment 0. 5 % Disease Free AC->TH 0 6 12 18 24 30 Months 36 42 48 54
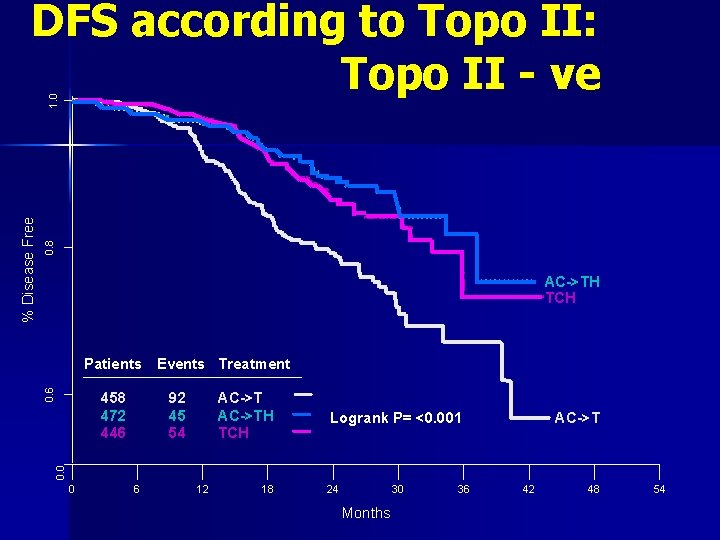
0. 8 AC->TH TCH 0. 6 Patients Events Treatment 458 472 446 92 45 54 AC->TH TCH Logrank P= <0. 001 AC->T 0. 0 % Disease Free 1. 0 DFS according to Topo II: Topo II - ve 0 6 12 18 24 30 Months 36 42 48 54
Conclusion: Outcome according to Topo II n Among Topo II+ve (35% case): Herceptin + Carbo Tax is inferior to Herceptin + Anththracyclines n Among Topo II-ve (65% cases): Herceptin + Carbo Tax is same as Herceptin + Anththracyclines

n Might 9 weeks of Herceptin be enough?
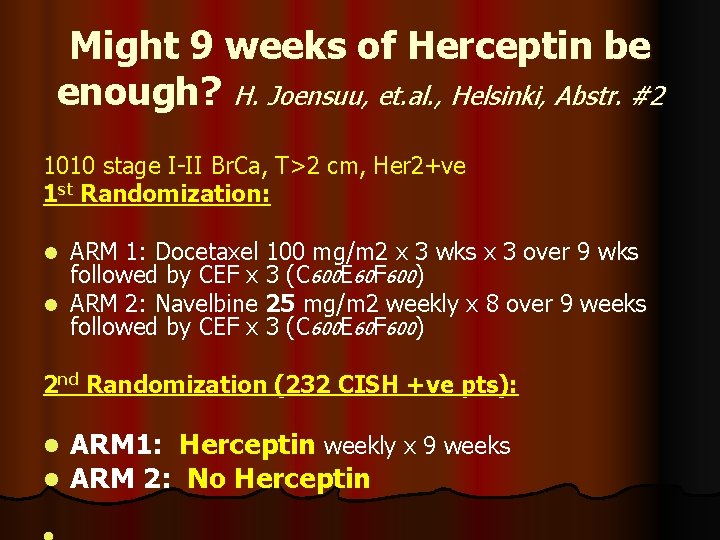
Might 9 weeks of Herceptin be enough? H. Joensuu, et. al. , Helsinki, Abstr. #2 1010 stage I-II Br. Ca, T>2 cm, Her 2+ve 1 st Randomization: ARM 1: Docetaxel 100 mg/m 2 x 3 wks x 3 over 9 wks followed by CEF x 3 (C 600 E 60 F 600) l ARM 2: Navelbine 25 mg/m 2 weekly x 8 over 9 weeks followed by CEF x 3 (C 600 E 60 F 600) l 2 nd Randomization (232 CISH +ve pts): l l ARM 1: Herceptin weekly x 9 weeks ARM 2: No Herceptin
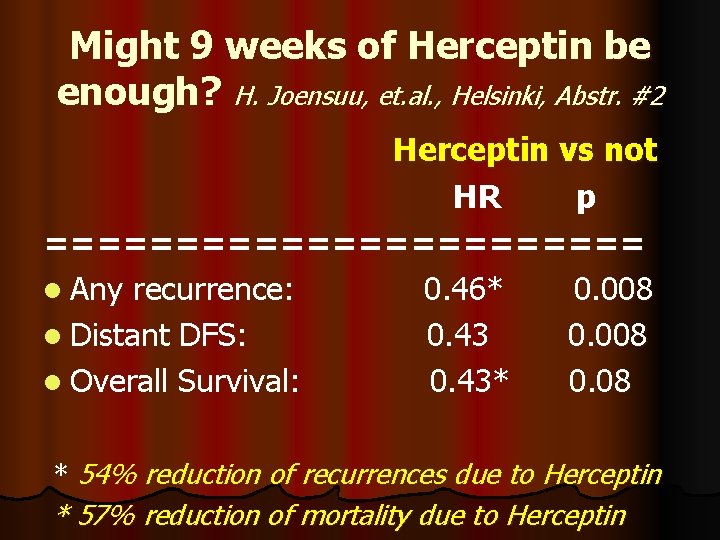
Might 9 weeks of Herceptin be enough? H. Joensuu, et. al. , Helsinki, Abstr. #2 Herceptin vs not HR p ============ l Any recurrence: 0. 46* 0. 008 l Distant DFS: 0. 43 0. 008 l Overall Survival: 0. 43* 0. 08 * 54% reduction of recurrences due to Herceptin * 57% reduction of mortality due to Herceptin
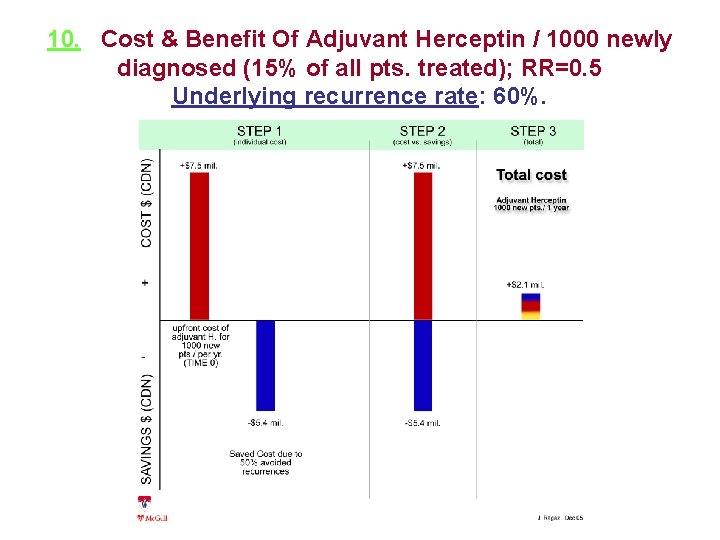
10. Cost & Benefit Of Adjuvant Herceptin / 1000 newly diagnosed (15% of all pts. treated); RR=0. 5 Underlying recurrence rate: 60%.
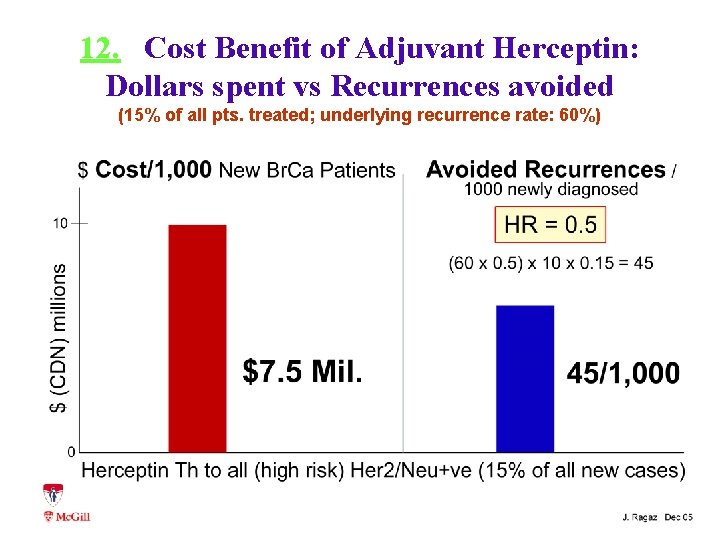
12. Cost Benefit of Adjuvant Herceptin: Dollars spent vs Recurrences avoided (15% of all pts. treated; underlying recurrence rate: 60%)
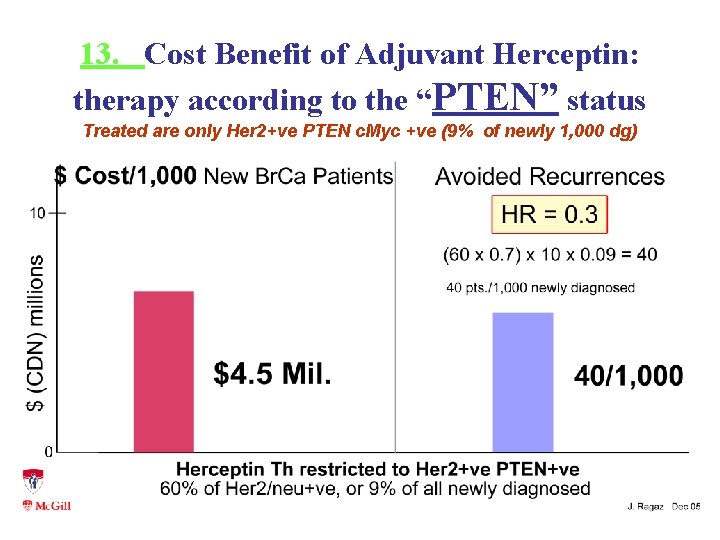
13. Cost Benefit of Adjuvant Herceptin: therapy according to the “PTEN” status Treated are only Her 2+ve PTEN c. Myc +ve (9% of newly 1, 000 dg)
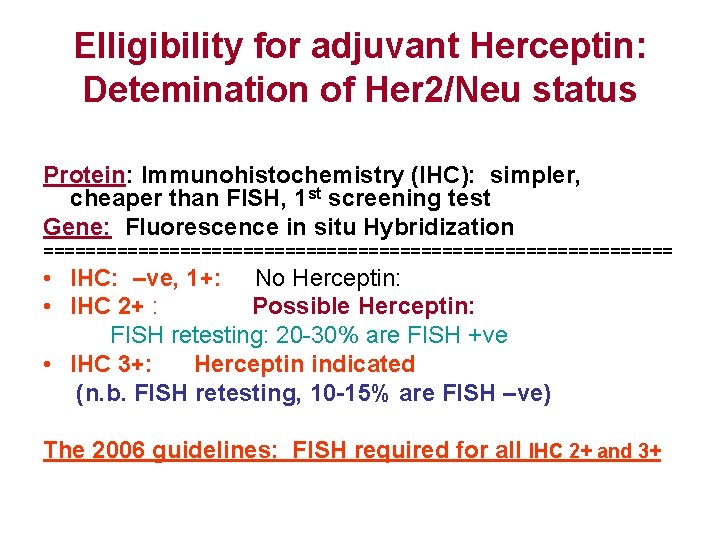
Elligibility for adjuvant Herceptin: Detemination of Her 2/Neu status Protein: Immunohistochemistry (IHC): simpler, cheaper than FISH, 1 st screening test Gene: Fluorescence in situ Hybridization ============================== • IHC: –ve, 1+: No Herceptin: • IHC 2+ : Possible Herceptin: FISH retesting: 20 -30% are FISH +ve • IHC 3+: Herceptin indicated (n. b. FISH retesting, 10 -15% are FISH –ve) The 2006 guidelines: FISH required for all IHC 2+ and 3+
Tissue Micro-Arrays: • Validation of new genes & proteins. . . • Stains of archival (paraffin embedded) tumor samples: • 300 pts / 1 plattform / long f/up…. Kononen J, Bubendorf L, Kallioniemi A, et al: Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nature Medicine 4: 844 -7, 1998

Her 2/Neu: FISH , presently “Gold Standard”…. • the red dots are her 2/neu gene copies. • greenish small dots are the cept 17/ chr D. Huntsman, L. Brown, BCCA

Her-2/Neu testing: FISH ADVANTAGES: l Close to 100% specific l 96. 5% sensitive l Low inter-laboratory variation DIS-ADVANTAGES: l High Cost and specialised equipment (Florescent microscopy) l Limited availability to community

Chromogenic in-situ hybridization CISH: advantages over FISH • Light microscope • Availability in the communities • Permanent stains (FISH stain fades after a few weeks) • Lower cost…
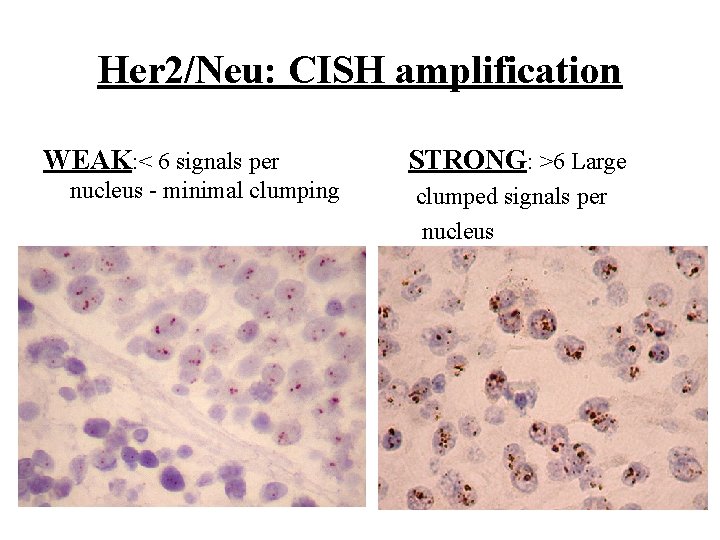
Her 2/Neu: CISH amplification WEAK: < 6 signals per nucleus - minimal clumping STRONG: >6 Large clumped signals per nucleus
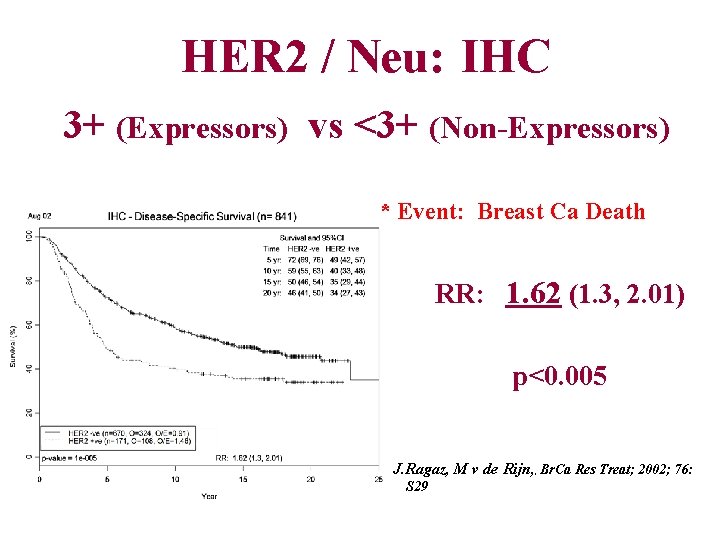
HER 2 / Neu: IHC 3+ (Expressors) vs <3+ (Non-Expressors) * Event: Breast Ca Death RR: 1. 62 (1. 3, 2. 01) p<0. 005 J. Ragaz, M v de Rijn, , Br. Ca Res Treat; 2002; 76: S 29
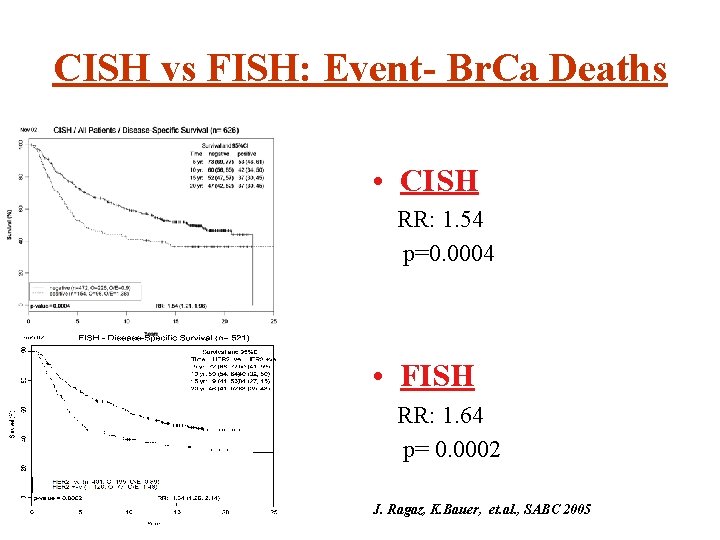
CISH vs FISH: Event- Br. Ca Deaths • CISH RR: 1. 54 p=0. 0004 • FISH RR: 1. 64 p= 0. 0002 J. Ragaz, K. Bauer, et. al. , SABC 2005

San Antonio 2006 l. Avastin
VEGF and Targeted Therapy: AVASTIN
Introduction l Several prior studies have shown association of Vascular Endothelial Growth Factor (VEGF) with human Breast Ca. However, only a few past studies had a sample size to measure outcome with sufficient power, or availability of multiple other markers. l In this study, impact of VEGF is correlated with the outcome in a large cohort of Br. Ca pts (N: 871) diagnosed between 1978 -1990. l

VEGF- HISTORY l l l 1960’s: J. Folkman, Neovascularization of malignant tumors Late 1980: VEGF was co-discovered (separately) by Hal Dvorak (who called it Vascular Permeability Factor) and Napoleone Ferrara. Late 1980 s: VEGF described as both an endothelial cell proliferative factor and as a compund increasing vascular permeability Early 1990’s: It was subsequently also found to be an important anti-apoptotic agent (G. Sledge et. al. ). Late 1990 s: K. Miller/ G. Sledge: Monoclonal antibody (bevacizumab/Avastin) went through Phase I testing.
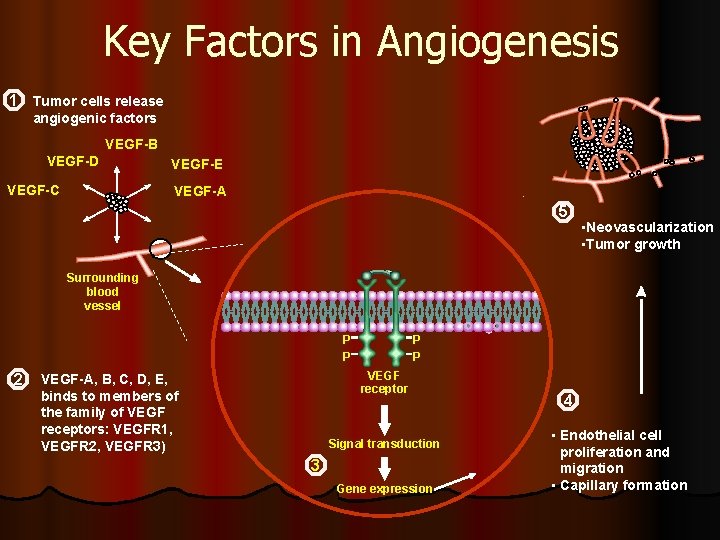
Key Factors in Angiogenesis 1 Tumor cells release angiogenic factors VEGF-B VEGF-D VEGF-C VEGF-E VEGF-A 5 • Neovascularization • Tumor growth Surrounding blood vessel P P 2 P P VEGF receptor VEGF-A, B, C, D, E, binds to members of the family of VEGF receptors: VEGFR 1, VEGFR 2, VEGFR 3) Signal transduction 3 Gene expression 4 • Endothelial cell proliferation and migration • Capillary formation
VEGF and Neo-Vascularization
VEGF and Neo-Vascularization
VEGF, Neo-Vascularization, Dissemination
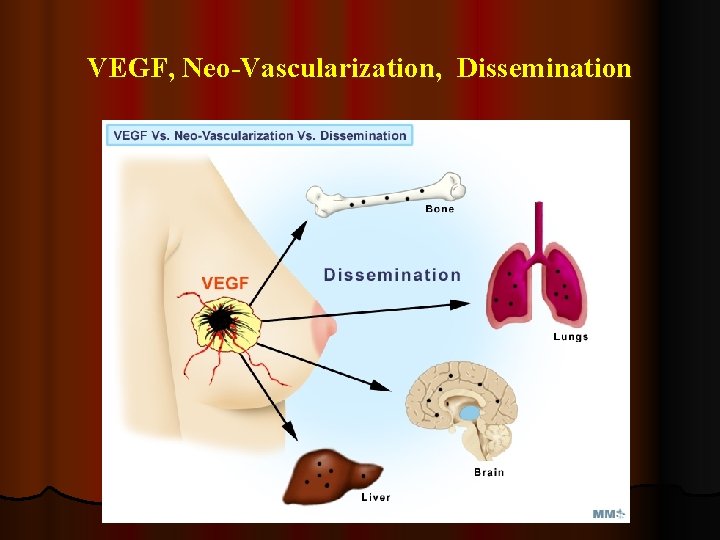
VEGF, Neo-Vascularization, Dissemination
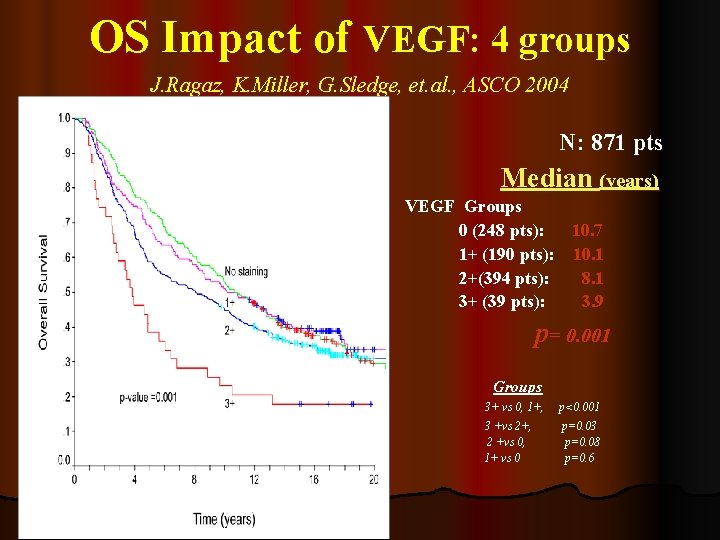
OS Impact of VEGF: 4 groups J. Ragaz, K. Miller, G. Sledge, et. al. , ASCO 2004 N: 871 pts Median (years) VEGF Groups 0 (248 pts): 10. 7 1+ (190 pts): 10. 1 2+(394 pts): 8. 1 3+ (39 pts): 3. 9 p= 0. 001 Groups 3+ vs 0, 1+, 3 +vs 2+, 2 +vs 0, 1+ vs 0 p<0. 001 p=0. 03 p=0. 08 p=0. 6
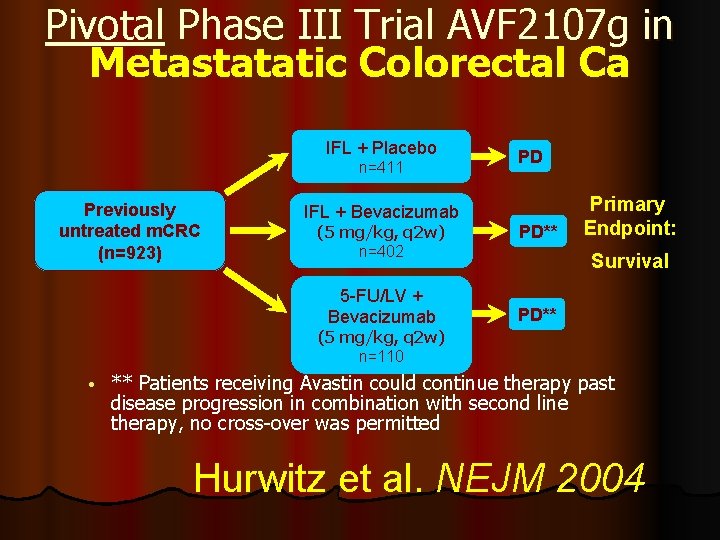
Pivotal Phase III Trial AVF 2107 g in Metastatatic Colorectal Ca IFL + Placebo n=411 Previously untreated m. CRC (n=923) IFL + Bevacizumab (5 mg/kg, q 2 w) n=402 5 -FU/LV + Bevacizumab PD PD** Primary Endpoint: Survival PD** (5 mg/kg, q 2 w) n=110 • ** Patients receiving Avastin could continue therapy past disease progression in combination with second line therapy, no cross-over was permitted Hurwitz et al. NEJM 2004
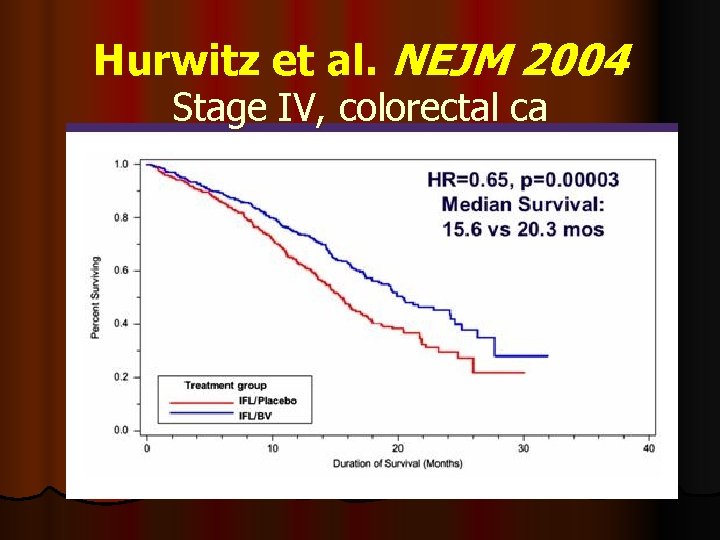
Hurwitz et al. NEJM 2004 Stage IV, colorectal ca

A randomised trial of paclitaxel versus paclitaxel plus bevacizumab (AVASTIN), as first-line therapy for stage IV Breast Ca KD Miller et. al, for ECOG 21000
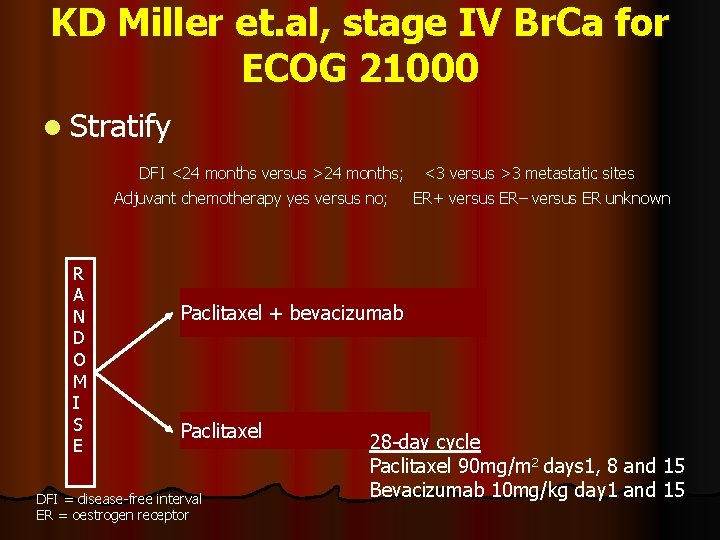
KD Miller et. al, stage IV Br. Ca for ECOG 21000 l Stratify DFI <24 months versus >24 months; Adjuvant chemotherapy yes versus no; R A N D O M I S E <3 versus >3 metastatic sites ER+ versus ER– versus ER unknown Paclitaxel + bevacizumab Paclitaxel DFI = disease-free interval ER = oestrogen receptor 28 -day cycle Paclitaxel 90 mg/m 2 days 1, 8 and 15 Bevacizumab 10 mg/kg day 1 and 15
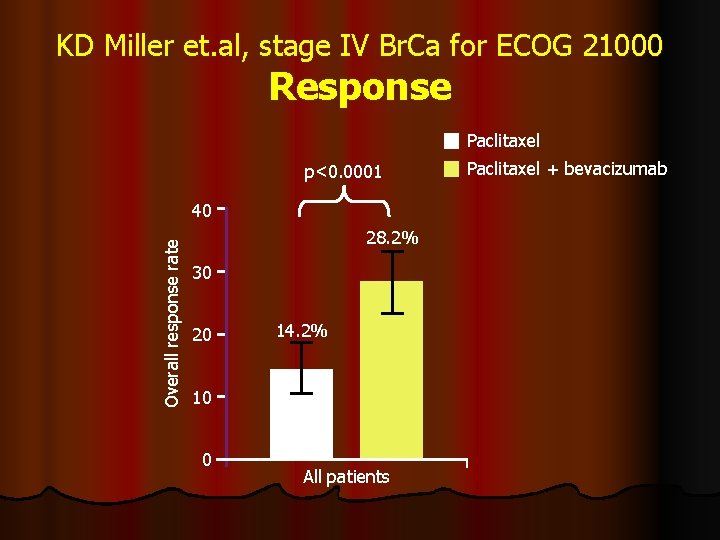
KD Miller et. al, stage IV Br. Ca for ECOG 21000 Response Paclitaxel p<0. 0001 Overall response rate 40 28. 2% 30 20 14. 2% 10 316 0 330 All patients Paclitaxel + bevacizumab
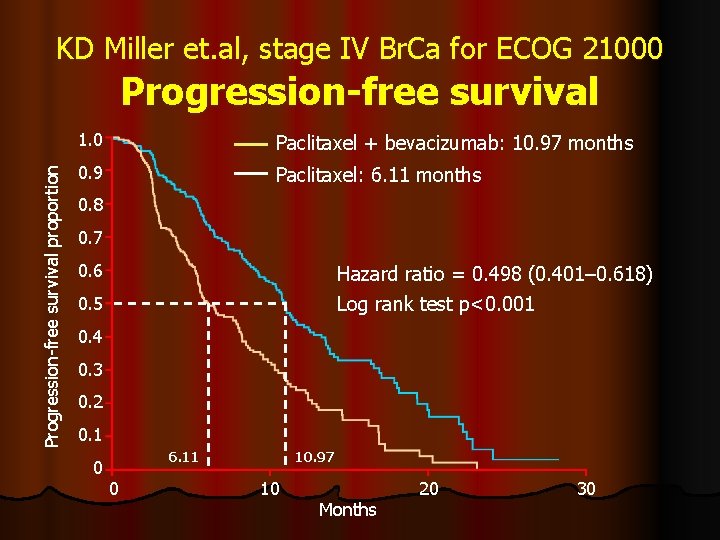
KD Miller et. al, stage IV Br. Ca for ECOG 21000 Progression-free survival proportion Progression-free survival 1. 0 Paclitaxel + bevacizumab: 10. 97 months 0. 9 Paclitaxel: 6. 11 months 0. 8 0. 7 0. 6 Hazard ratio = 0. 498 (0. 401– 0. 618) Log rank test p<0. 001 0. 5 0. 4 0. 3 0. 2 0. 1 0 6. 11 0 10. 97 10 Months 20 30
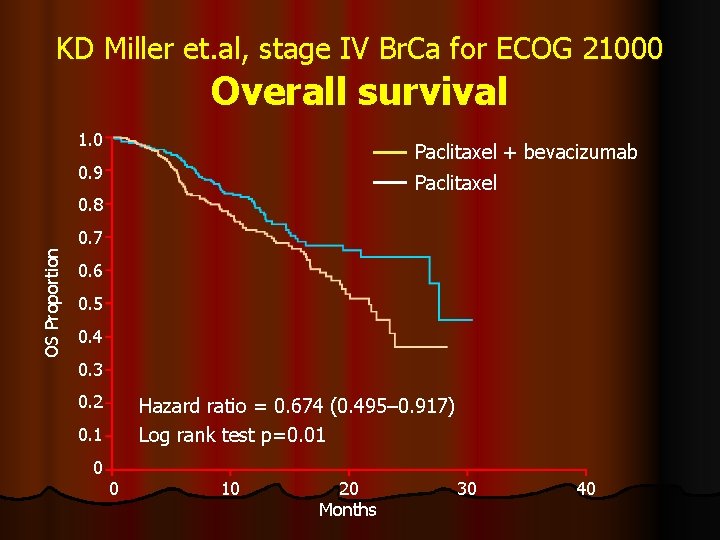
KD Miller et. al, stage IV Br. Ca for ECOG 21000 Overall survival 1. 0 Paclitaxel + bevacizumab 0. 9 Paclitaxel OS Proportion 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 Hazard ratio = 0. 674 (0. 495– 0. 917) Log rank test p=0. 01 0. 1 0 0 10 20 Months 30 40
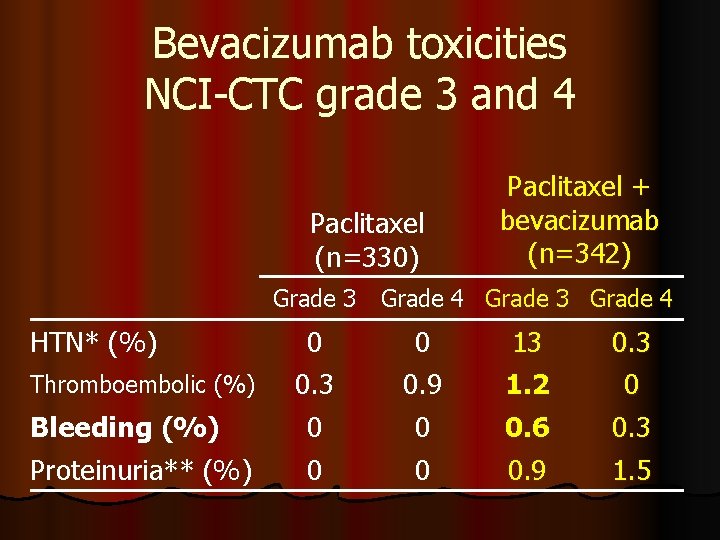
Bevacizumab toxicities NCI-CTC grade 3 and 4 Paclitaxel (n=330) Paclitaxel + bevacizumab (n=342) Grade 3 Grade 4 HTN* (%) Thromboembolic (%) 0 0. 3 0 0. 9 13 1. 2 0. 3 0 Bleeding (%) Proteinuria** (%) 0 0 0. 6 0. 9 0. 3 1. 5
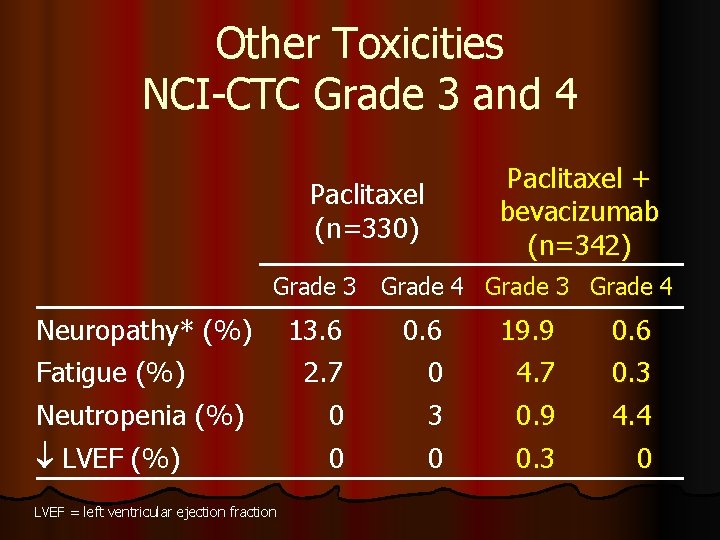
Other Toxicities NCI-CTC Grade 3 and 4 Paclitaxel + bevacizumab (n=342) Paclitaxel (n=330) Grade 3 Grade 4 Neuropathy* (%) Fatigue (%) 13. 6 2. 7 0. 6 0 19. 9 4. 7 0. 6 0. 3 Neutropenia (%) LVEF (%) 0 0 3 0 0. 9 0. 3 4. 4 0 LVEF = left ventricular ejection fraction
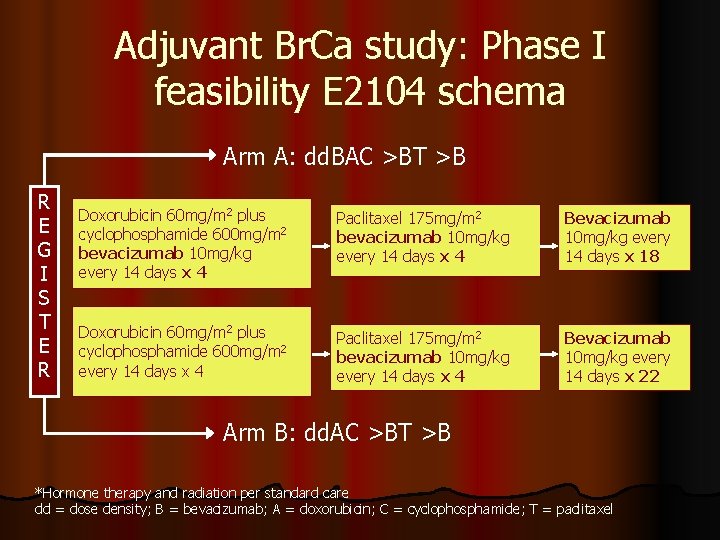
Adjuvant Br. Ca study: Phase I feasibility E 2104 schema Arm A: dd. BAC >BT >B R E G I S T E R Doxorubicin 60 mg/m 2 plus cyclophosphamide 600 mg/m 2 bevacizumab 10 mg/kg every 14 days x 4 Paclitaxel 175 mg/m 2 bevacizumab 10 mg/kg every 14 days x 4 Bevacizumab 10 mg/kg every 14 days x 18 Doxorubicin 60 mg/m 2 plus cyclophosphamide 600 mg/m 2 every 14 days x 4 Paclitaxel 175 mg/m 2 bevacizumab 10 mg/kg every 14 days x 4 Bevacizumab 10 mg/kg every 14 days x 22 Arm B: dd. AC >BT >B *Hormone therapy and radiation per standard care dd = dose density; B = bevacizumab; A = doxorubicin; C = cyclophosphamide; T = paclitaxel
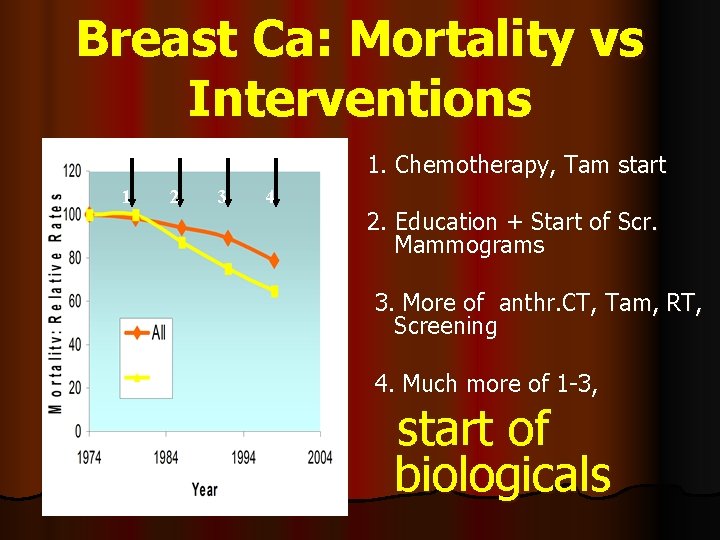
Breast Ca: Mortality vs Interventions 1. Chemotherapy, Tam start 1 2 3 4 2. Education + Start of Scr. Mammograms 3. More of anthr. CT, Tam, RT, Screening 4. Much more of 1 -3, start of biologicals

21

Thank You Joseph Ragaz n Tel. : (514) 843 - 1527 n Joseph. ragaz@mcgill. ca n
- Slides: 77