The Bohr Model the EM Spectrum Mrs Morris
-The Bohr Model & the EM Spectrum Mrs. Morris Chemistry
Dalton’s Atomic Model n Billiard Ball

Thompson’s Model n Plum Pudding (chocolate chip cookie dough)
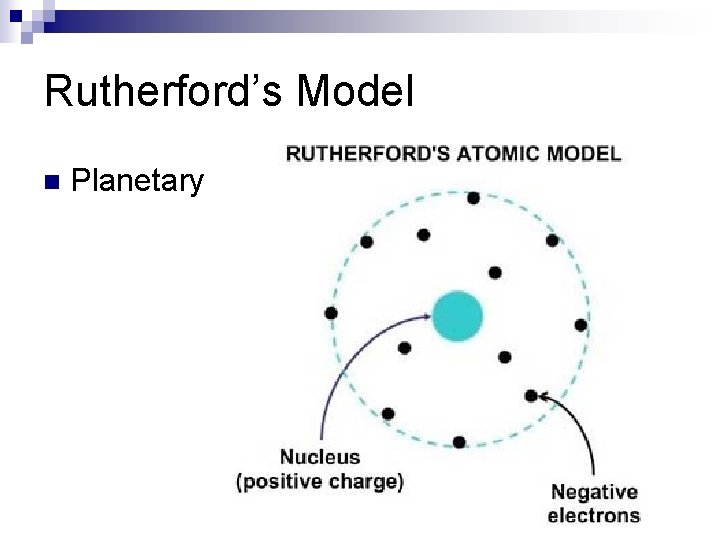
Rutherford’s Model n Planetary
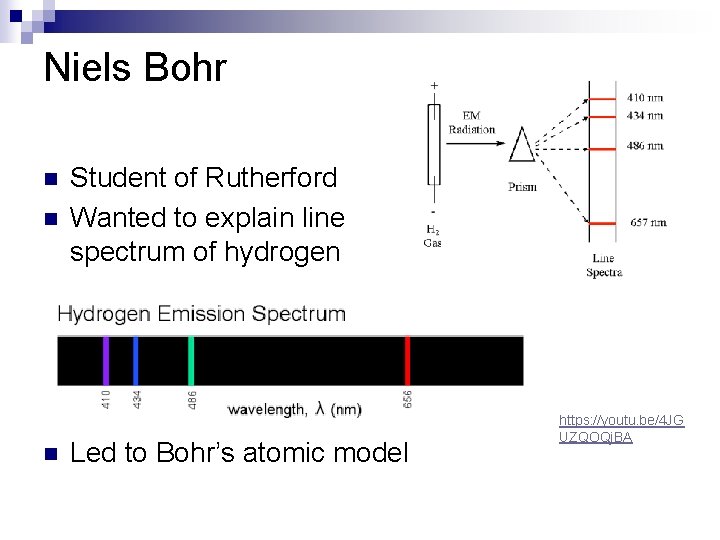
Niels Bohr n n n Student of Rutherford Wanted to explain line spectrum of hydrogen Led to Bohr’s atomic model https: //youtu. be/4 JG UZQOQj. BA
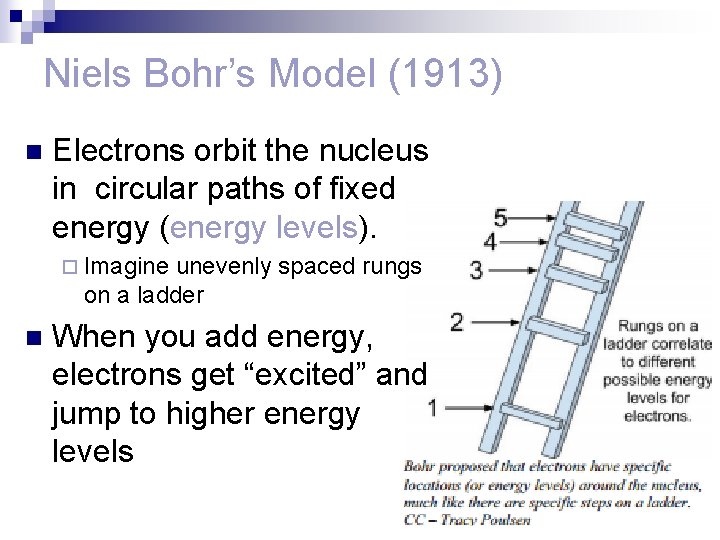
Niels Bohr’s Model (1913) n Electrons orbit the nucleus in circular paths of fixed energy (energy levels). ¨ Imagine unevenly spaced rungs on a ladder n When you add energy, electrons get “excited” and jump to higher energy levels
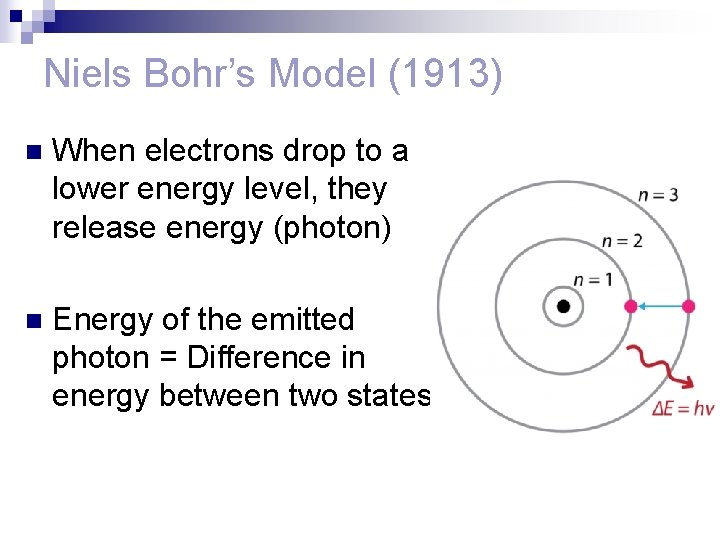
Niels Bohr’s Model (1913) n When electrons drop to a lower energy level, they release energy (photon) n Energy of the emitted photon = Difference in energy between two states

Excited State and Ground State n Ground state: the lowest possible energy level an electron be at. n Excited state: an energy level higher than the ground state.
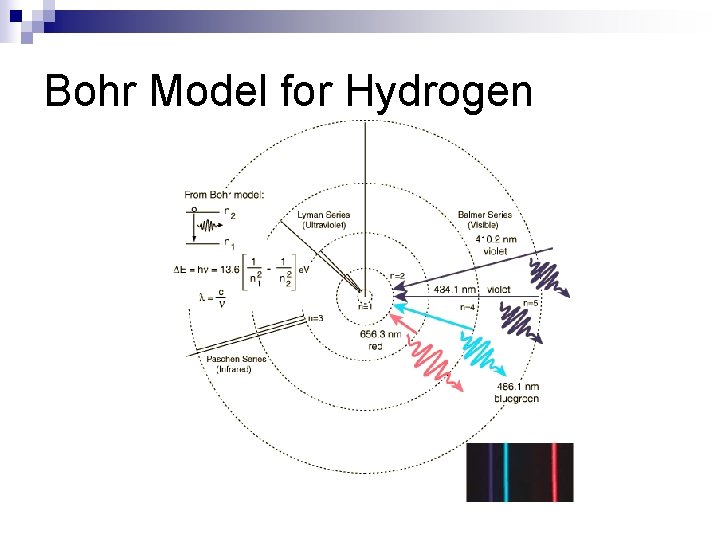
Bohr Model for Hydrogen
Limitations n The Bohr model explained the emission spectrum of the hydrogen atom but did not always explain those of other elements.
Standards n SC. 912. P. 10. 9: Describe the quantization of energy at the atomic level. ¨ describe the quantization of energy through the behavior of electrons changing energy levels, including: n electrons absorbing energy move to higher (excited) energy states and releasing energy returns them to a lower or ground state ¨ describe the Bohr model and the quantum mechanical model of the atom
- Slides: 11