The Bohr Model Chemistry Daltons Atomic Model Plum

-The Bohr Model Chemistry

Dalton’s Atomic Model

Plum Pudding Model (Thomson)
Niels Bohr (Born in Denmark 1885 -1962) n Student of Rutherford
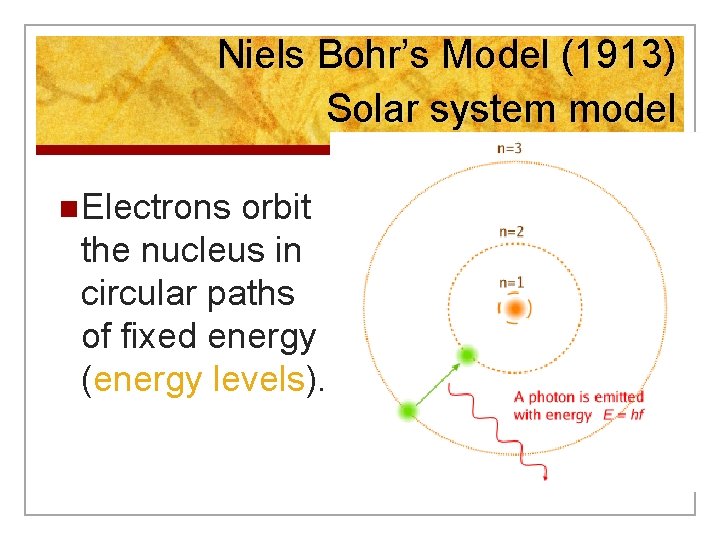
Niels Bohr’s Model (1913) Solar system model n Electrons orbit the nucleus in circular paths of fixed energy (energy levels).
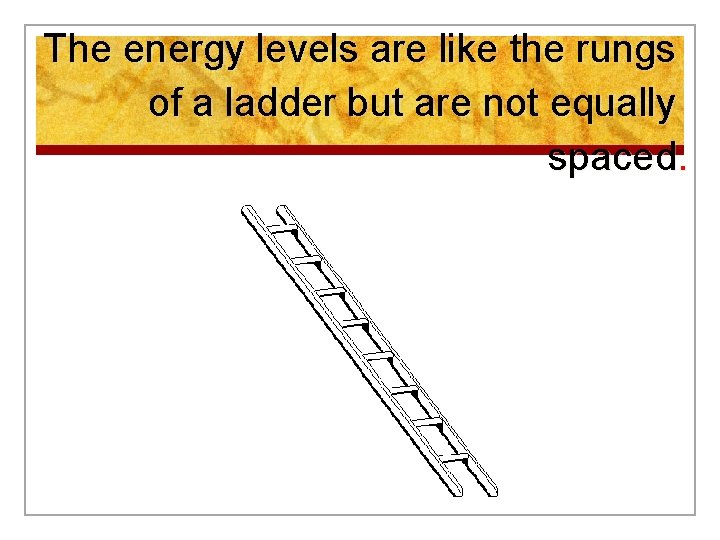
The energy levels are like the rungs of a ladder but are not equally spaced.

Electrons in energy levels closest to nucleus have the least amount of energy
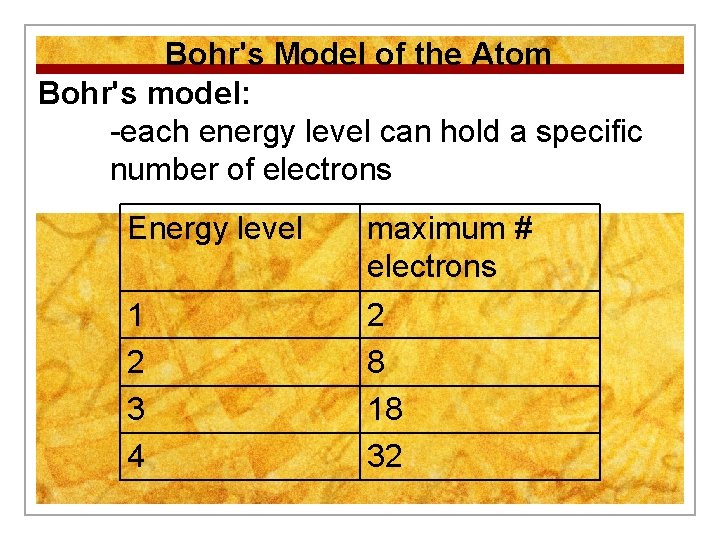
Bohr's Model of the Atom Bohr's model: -each energy level can hold a specific number of electrons Energy level maximum # electrons 1 2 3 4 2 8 18 32
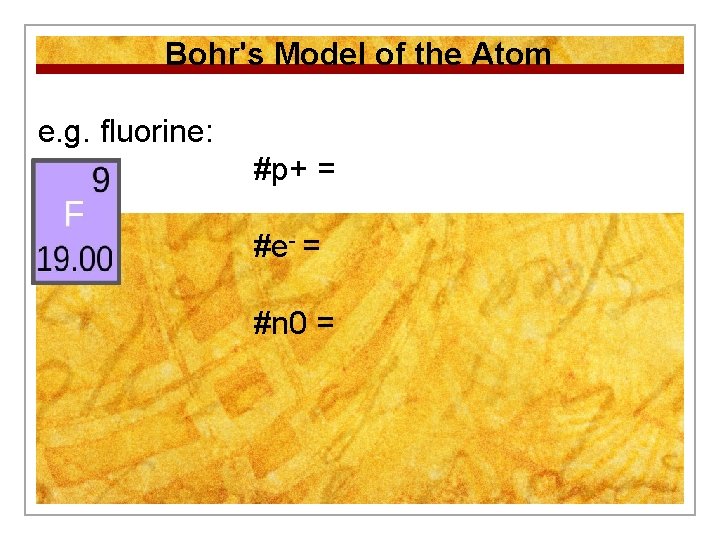
Bohr's Model of the Atom e. g. fluorine: #p+ = #e- = #n 0 =
Shorthand form of Bohr’s model

Assignment n. Draw the Bohr models for elements 1 -20 (shorthand)

Excited State and Ground State n. Ground state: the lowest possible energy level occupied by an electron when it is most stable n. Excited state: an energy level higher than the ground state.

Niels Bohr’s Atom Cont’d n Electrons can jump from energy level to energy level. n Electrons absorb or emit light energy when they jump from one energy level to another.

Quantum n. A quantum of energy is the amount of energy required to move an electron from one energy level to another.
Photons n Photons are bundles of light energy that are emitted by electrons as they go from higher energy levels to lower levels.

n Energy emitted by the electron as it leaps from the higher to the lower energy level is proportional to the frequency of the light wave. n Frequency defines the color of visible light.

Emission Spectrum n Light emitted produces a unique emission spectrum. n We use a spectroscope to observe the spectrum
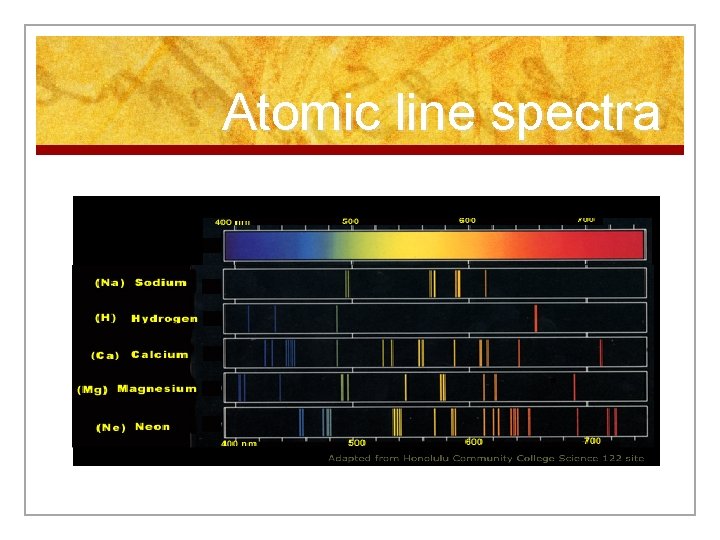
Atomic line spectra
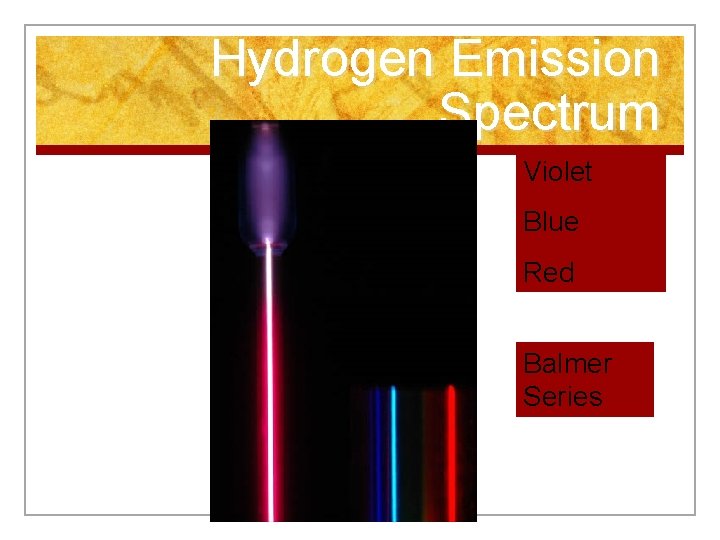
Hydrogen Emission Spectrum Violet Blue Red Balmer Series
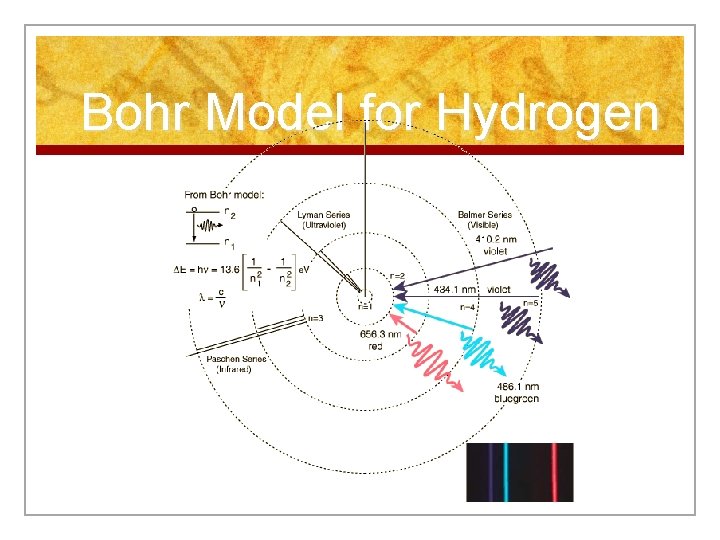
Bohr Model for Hydrogen
- Slides: 21