The Bohr Model BrightLine Spectra Take notes on
The Bohr Model & Bright-Line Spectra Take notes on what is written in blue…
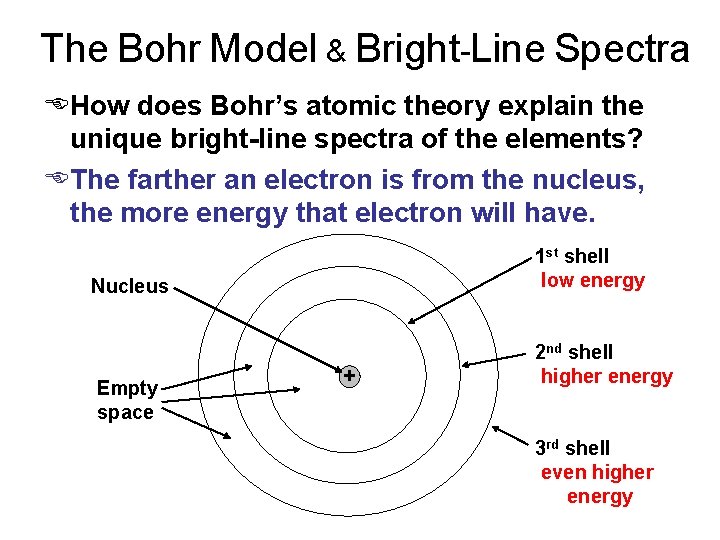
The Bohr Model & Bright-Line Spectra EHow does Bohr’s atomic theory explain the unique bright-line spectra of the elements? EThe farther an electron is from the nucleus, the more energy that electron will have. 1 st shell low energy Nucleus Empty space + 2 nd shell higher energy 3 rd shell even higher energy
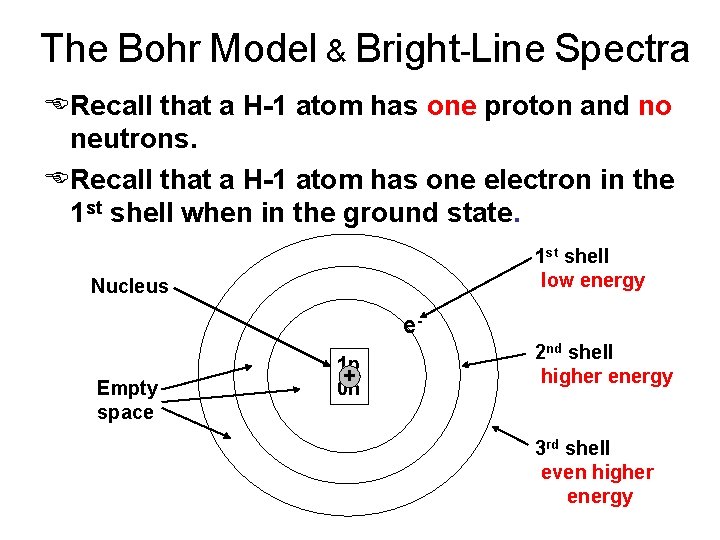
The Bohr Model & Bright-Line Spectra ERecall that a H-1 atom has one proton and no neutrons. ERecall that a H-1 atom has one electron in the 1 st shell when in the ground state. 1 st shell low energy Nucleus e. Empty space 1 p + 0 n 2 nd shell higher energy 3 rd shell even higher energy
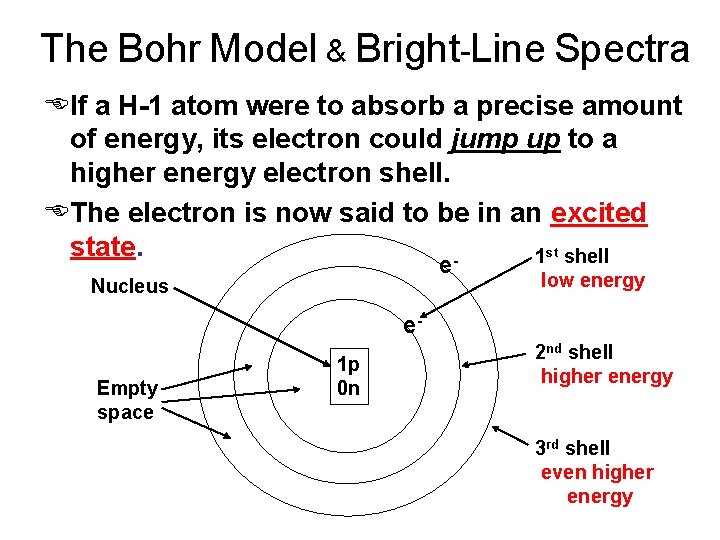
The Bohr Model & Bright-Line Spectra EIf a H-1 atom were to absorb a precise amount of energy, its electron could jump up to a higher energy electron shell. EThe electron is now said to be in an excited state. 1 st shell e Nucleus low energy e. Empty space 1 p 0 n 2 nd shell higher energy 3 rd shell even higher energy

The Bohr Model & Bright-Line Spectra (Write this down…) EElectrons are in the excited state when they in a shell that is higher than their ground state. EElectrons can only jump up to an excited state by absorbing a precise amount of energy. EAll electrons in the excited state will return to their ground state. EElectrons can NEVER jump to a shell that is lower than their ground state.
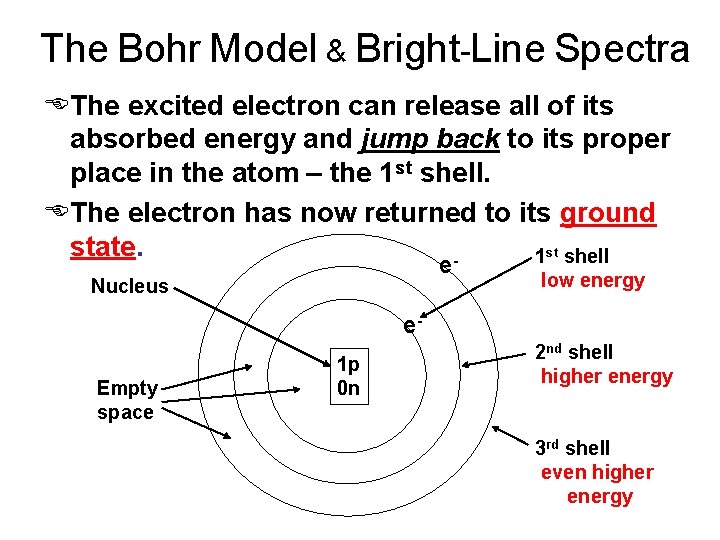
The Bohr Model & Bright-Line Spectra EThe excited electron can release all of its absorbed energy and jump back to its proper place in the atom – the 1 st shell. EThe electron has now returned to its ground state. 1 st shell e Nucleus low energy e. Empty space 1 p 0 n 2 nd shell higher energy 3 rd shell even higher energy
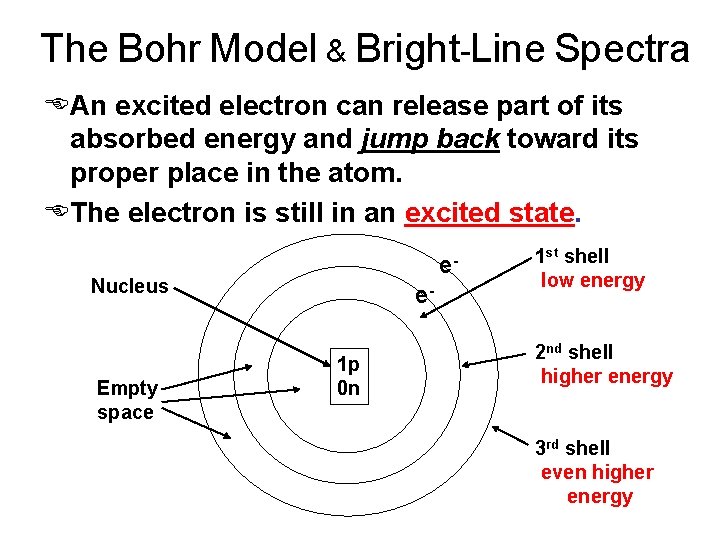
The Bohr Model & Bright-Line Spectra EAn excited electron can release part of its absorbed energy and jump back toward its proper place in the atom. EThe electron is still in an excited state. e- Nucleus Empty space e 1 p 0 n 1 st shell low energy 2 nd shell higher energy 3 rd shell even higher energy
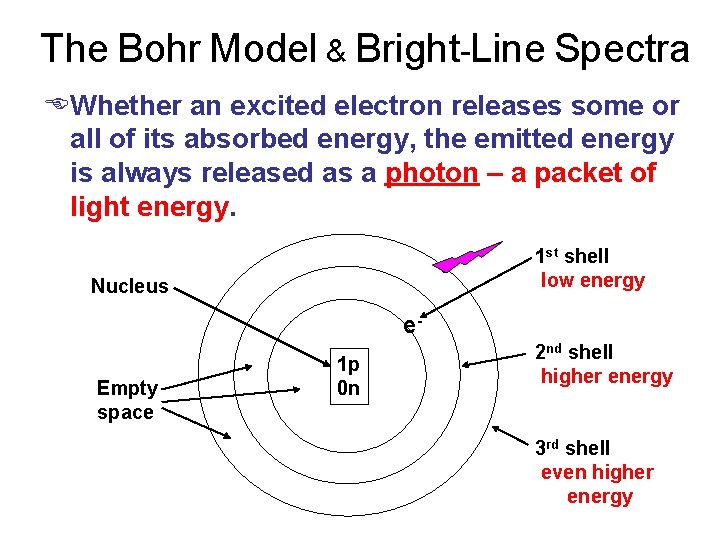
The Bohr Model & Bright-Line Spectra EWhether an excited electron releases some or all of its absorbed energy, the emitted energy is always released as a photon – a packet of light energy. e- Nucleus 1 st shell low energy e. Empty space 1 p 0 n 2 nd shell higher energy 3 rd shell even higher energy
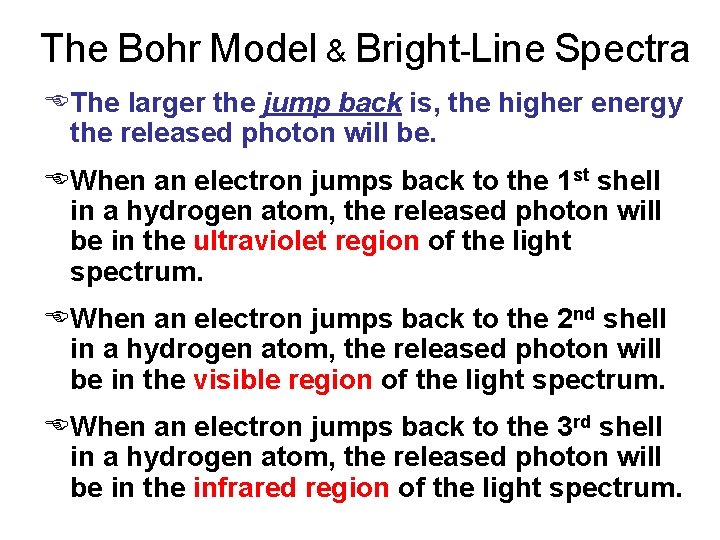
The Bohr Model & Bright-Line Spectra EThe larger the jump back is, the higher energy the released photon will be. EWhen an electron jumps back to the 1 st shell in a hydrogen atom, the released photon will be in the ultraviolet region of the light spectrum. EWhen an electron jumps back to the 2 nd shell in a hydrogen atom, the released photon will be in the visible region of the light spectrum. EWhen an electron jumps back to the 3 rd shell in a hydrogen atom, the released photon will be in the infrared region of the light spectrum.

The Bohr Model & Bright-Line Spectra EThe four lines of the visible bright-line spectrum for a sample of hydrogen are produced by the following electron jumps. An electron jumping from the 5 th shell to the 2 nd shell An electron jumping from the 6 th shell to the 2 nd shell An electron jumping from the 3 rd shell to the 2 nd shell An electron jumping from the 4 th shell to the 2 nd shell hydrogen 400 nm 500 nm 700 nm
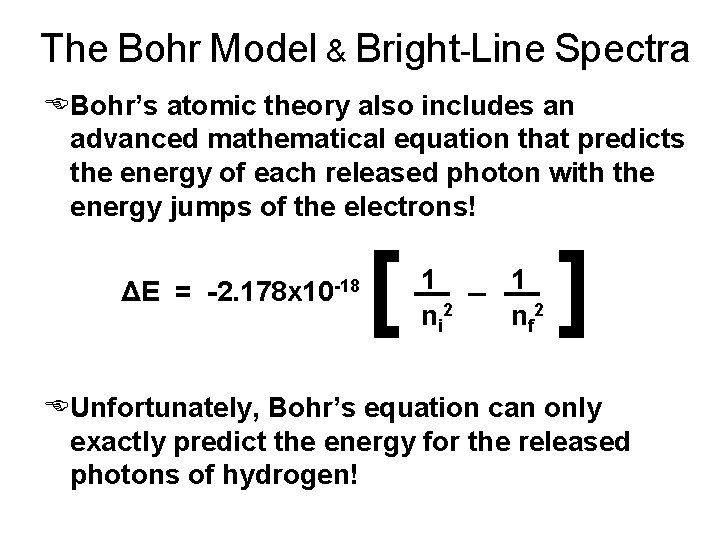
The Bohr Model & Bright-Line Spectra EBohr’s atomic theory also includes an advanced mathematical equation that predicts the energy of each released photon with the energy jumps of the electrons! ΔE = -2. 178 x 10 -18 [ 1 n i 2 1 n f 2 ] EUnfortunately, Bohr’s equation can only exactly predict the energy for the released photons of hydrogen!
The Bohr Model & Bright-Line Spectra EEven though Bohr’s atomic model has some serious flaws, it is still used to explain many chemical phenomena. Ex. 1) Which electron shell has the most energy? (1) 1 st shell (2) 2 nd shell (3) 3 rd shell (4) 4 th shell Ex. 2) An electron becomes excited by (1) absorbing energy (2) releasing energy (3) producing energy (4) destroying energy
The Bohr Model & Bright-Line Spectra Ex. 5) In order for an excited state electron to return to the ground state it must (1) absorb energy (2) release energy (3) store energy (4) destroy energy Ex. 6) A line in an element’s bright-line spectrum is produced when (1) a ground state electron absorbs energy (2) an excited state electron releases energy (3) an excited state electron absorbs energy (4) a ground state electron releases energy

The Bohr Model & Bright-Line Spectra Ex. 7) Which of the following types of light has the most energy? (1) radio waves (2) infrared light (3) visible light (4) ultraviolet light Ex. 8) Which of the following colors in the visible spectrum has the most energy? (1) red (2) violet (3) yellow (4) blue R O Y G B V
The Bohr Model & Bright-Line Spectra Ex. 9) A bundle of light energy is called a(n) (1) photon (2) electron (3) wave (4) quark Ex. 10) In order for a ground state electron to jump up to a higher shell and become an excited state electron, it must (1) absorb an inexact amount energy (2) release an inexact amount energy (3) absorb a precise amount of energy (4) release a precise amount of energy

Assignment • SA 5. 3 page 146 #16 -21
- Slides: 16