The Biomedical Translational Research Information System BTRIS James
The Biomedical Translational Research Information System (BTRIS) James J. Cimino Chief, Laboratory for Informatics Development National Institutes of Health Clinical Center Senior Scientist, Lister Hill Center for Biomedical Communications National Library of Medicine Adjunct Professor, Department of Biomedical Informatics Columbia University College of Physicians and Surgeons September 12, 2008 B RIS

Learning Objectives 1. An introduction to the compilation and integration of clinical and research data from multiple disparate sources 2. Understanding the authorization issues related to reuse of patient clinical data 3. Understanding the terminology issues related to the reuse of coded clinical and research data 4. Familiarity with the approach being taken at the National Institutes of Health to collect, integrate, and code clinical and research data into a single repository, for authorized B RIS resuse in biomedical research.

No Relevant Financial Relationships with Commercial Interests B RIS

The Clinical Center, 1981 B RIS
What Was the Problem? • Patients in the NIH Clinical Center were on research protocols, but… • …their data were in a clinical information system • Needed to extract individual data for analysis • Needed cross-patient queries for additional analysis B RIS
Patient Information Graphics System (PIGS) • Integration of a desktop computer (pre-PC) to mainframe clinical information system (TDS) • Download vital sign and drug administration data • Various graphical displays • SCAMC, 1981 B RIS

B RIS

B RIS

B RIS

The Columbia University Medical Center Clinical Data Warehouse • Clinical data repository to collect data from ancillary systems • Coded with the Medical Entities Dictionary B RIS
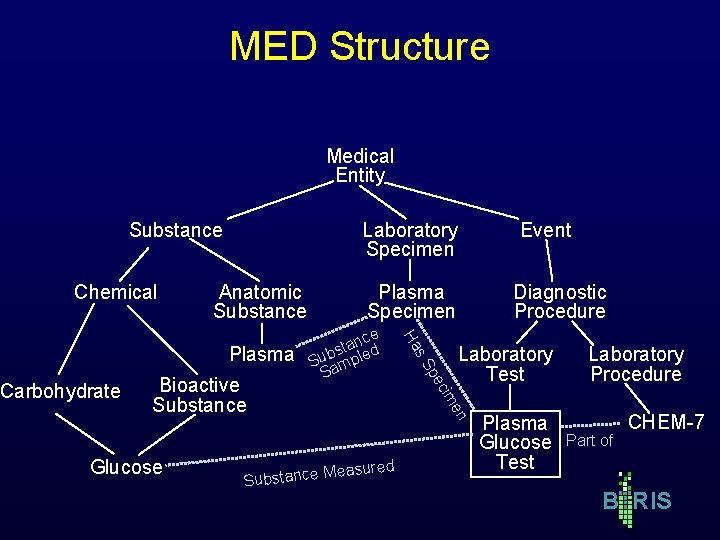
MED Structure Medical Entity Substance Chemical Laboratory Specimen Anatomic Substance asured e M e c n ta s Sub Laboratory Test en Glucose Diagnostic Procedure Laboratory Procedure im Bioactive Substance c pe Carbohydrate nce a t s d Subample S s. S Ha Plasma Specimen Event Plasma Glucose Test Part of CHEM-7 B RIS
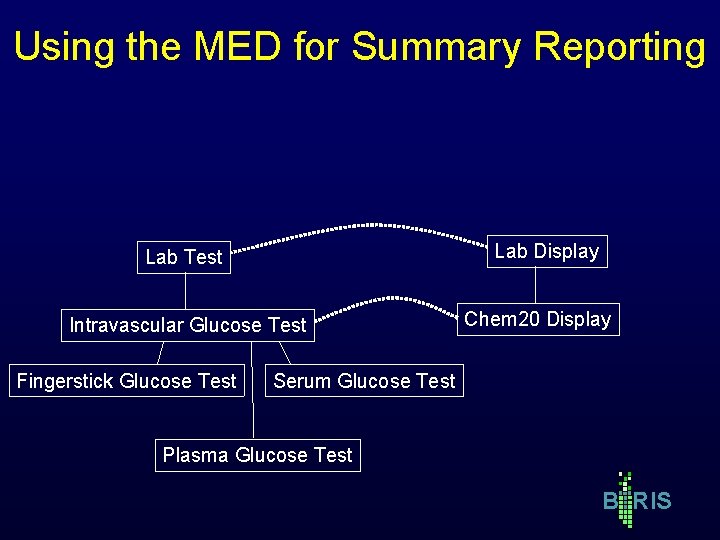
Using the MED for Summary Reporting Lab Display Lab Test Intravascular Glucose Test Fingerstick Glucose Test Chem 20 Display Serum Glucose Test Plasma Glucose Test B RIS

The Columbia University Medical Center Clinical Data Warehouse • Clinical data repository to collect data from ancillary systems • Coded with the Medical Entities Dictionary • Back end for clinical information systems (CIS, Web. CIS, Pat. CIS, Palm. CIS, Qing. CIS, MendonÇIS…) • Reorganized for use as Clinical Data Warehouse B RIS
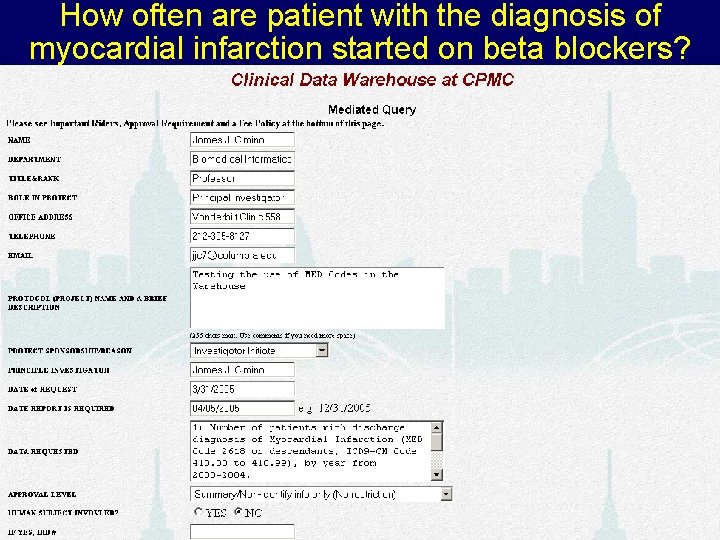
How often are patient with the diagnosis of myocardial infarction started on beta blockers? B RIS
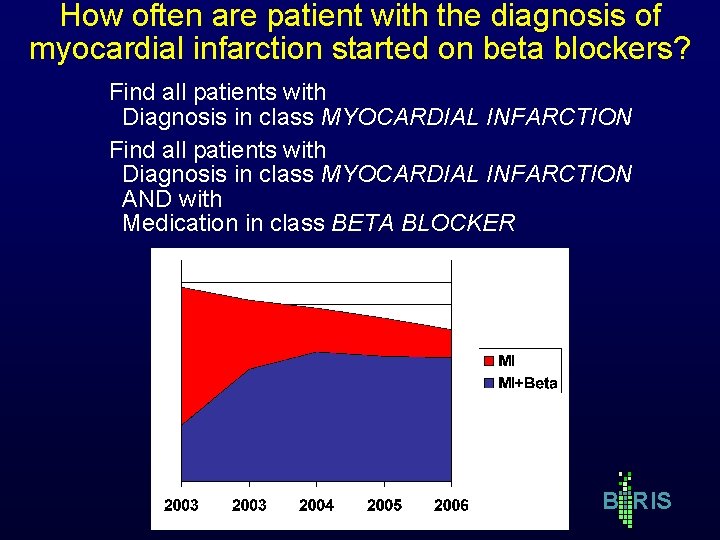
How often are patient with the diagnosis of myocardial infarction started on beta blockers? Find all patients with Diagnosis in class MYOCARDIAL INFARCTION AND with Medication in class BETA BLOCKER B RIS

The Clinical Center, 2008 B RIS
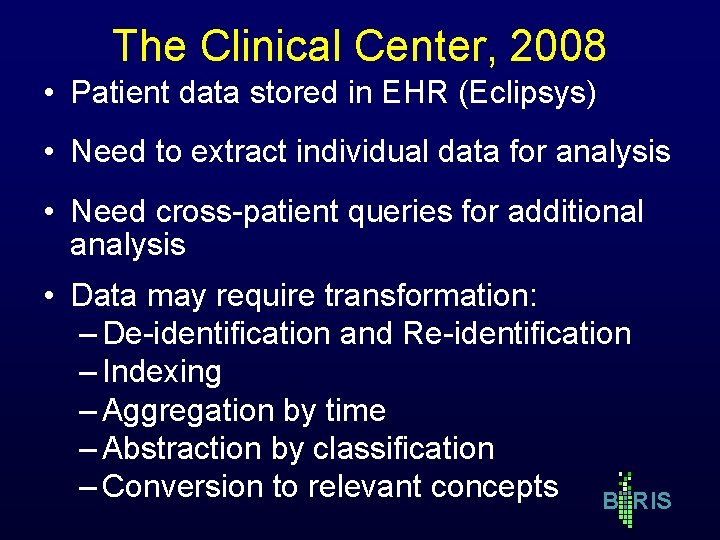
The Clinical Center, 2008 • Patient data stored in EHR (Eclipsys) • Need to extract individual data for analysis • Need cross-patient queries for additional analysis • Data may require transformation: – De-identification and Re-identification – Indexing – Aggregation by time – Abstraction by classification – Conversion to relevant concepts B RIS
What is BTRIS? • Biomedical data – Research data collected using clinical information systems – Clinical data collected using clinical information systems – Research data from research information systems – Non-human data • Reuse of data to support translational research • Hence: Biomedical Translational Research Information System B RIS
The NIH, 2008 B RIS
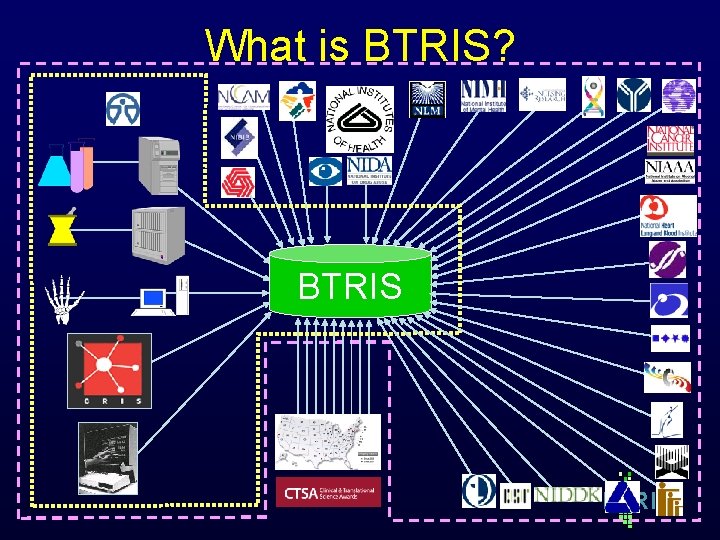
What is BTRIS? BTRIS B RIS
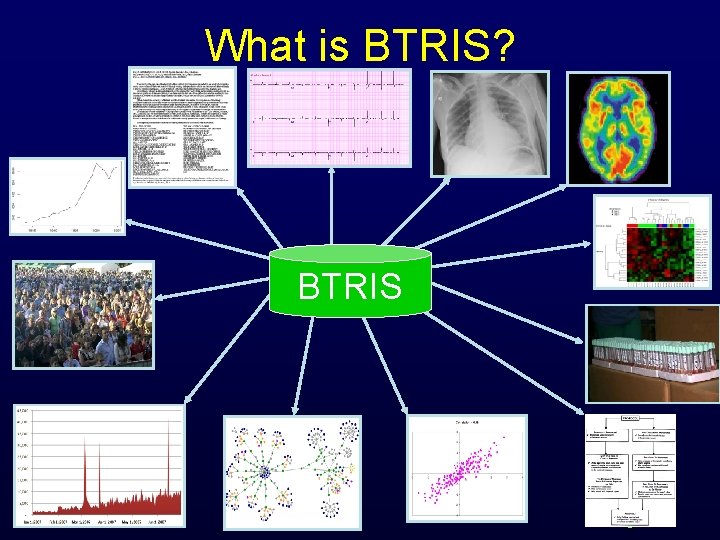
What is BTRIS? BTRIS B RIS
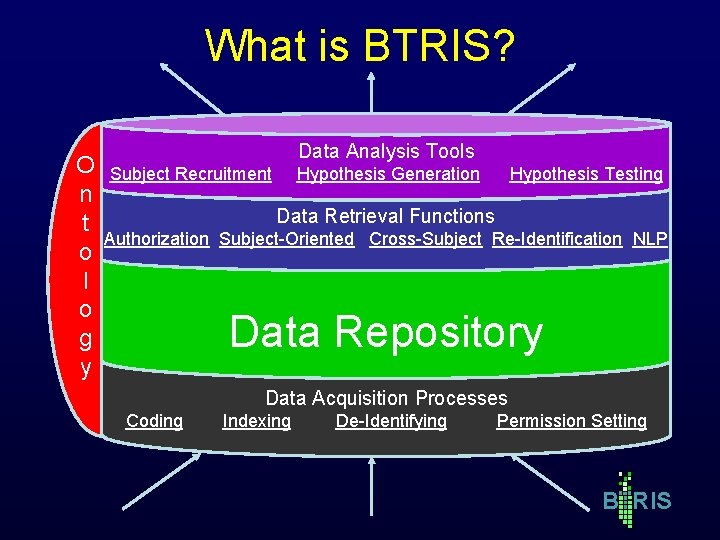
What is BTRIS? O n t o l o g y Data Analysis Tools Subject Recruitment Hypothesis Generation Hypothesis Testing Data Retrieval Functions Authorization Subject-Oriented Cross-Subject Re-Identification NLP BTRIS Data Repository Data Acquisition Processes Coding Indexing De-Identifying Permission Setting B RIS
BTRIS Issues • Data sources • Data model integration • Research Entities Dictionary • Access policies • User requirements • User access and user Interface • Terminology-based queries B RIS
Data Sources • Order entry system • Ancillary systems • Institute and Center (IC) systems • Individual researchers’ systems • Notebooks B RIS
Data Model Integration • Events and details • Entity-relation vs entity-attribute-value • Denormalization B RIS

Research Entities Dictionary • Apelon’s Terminology Development Editor • NCI/ca. BIG Thesaurus B RIS
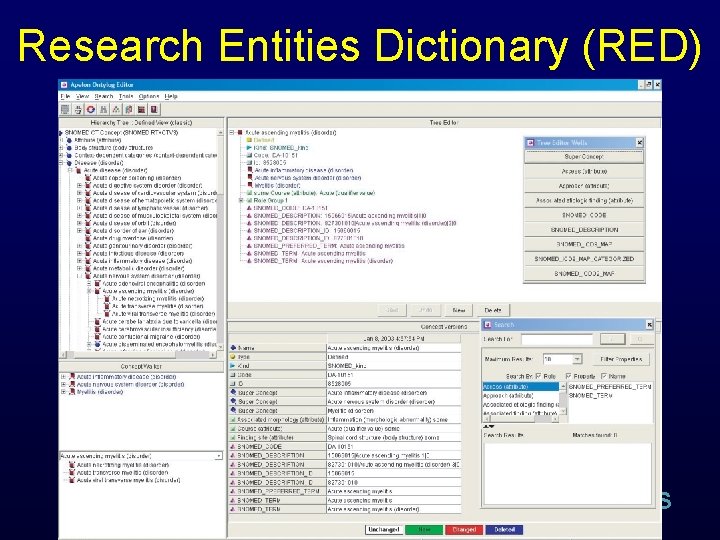
Research Entities Dictionary (RED) B RIS

Research Entities Dictionary • Apelon’s Terminology Development Editor • NCI/ca. BIG Thesaurus • Content • Organization B RIS

Access Policies • Privacy Act, not HIPAA • Policy Working Group • Intellectual property vs. public domain • Identifiers • Unlinked, coded data B RIS
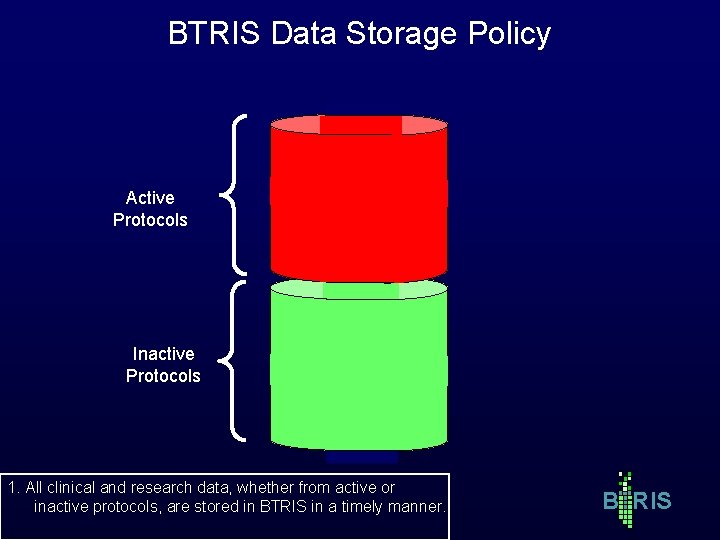
BTRIS Data Storage Policy Active Protocols Inactive Protocols 1. All clinical and research data, whether from active or inactive protocols, are stored in BTRIS in a timely manner. B RIS
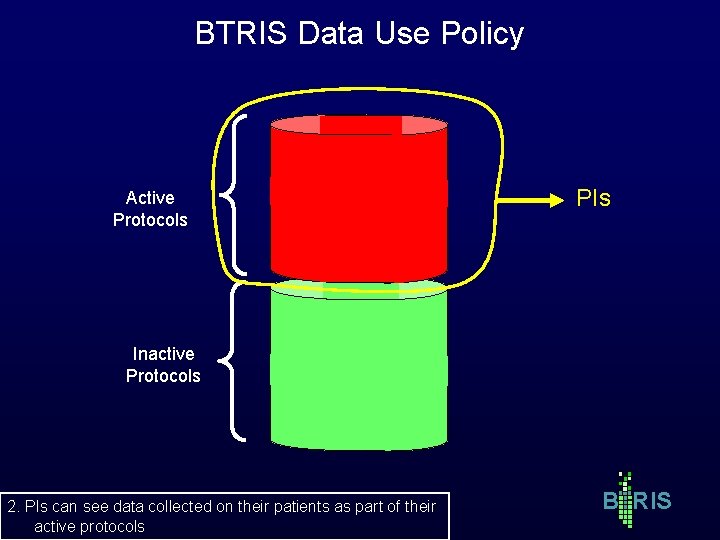
BTRIS Data Use Policy Active Protocols PIs Inactive Protocols 2. PIs can see data collected on their patients as part of their active protocols B RIS
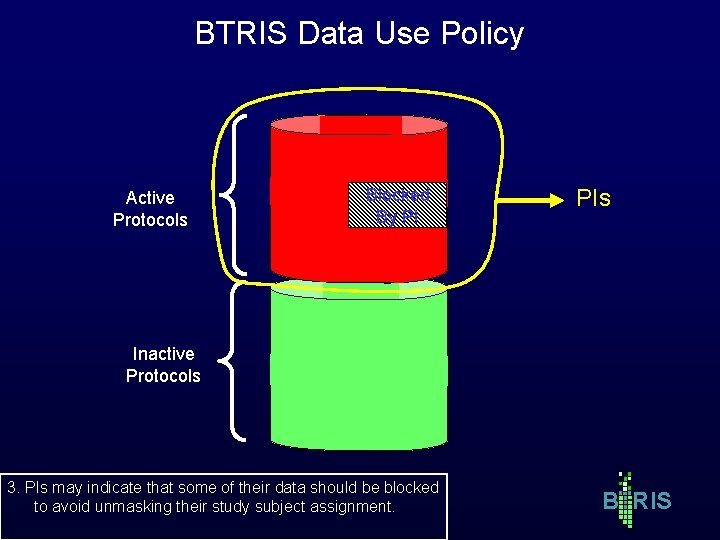
BTRIS Data Use Policy Active Protocols Blocked By PI PIs Inactive Protocols 3. PIs may indicate that some of their data should be blocked to avoid unmasking their study subject assignment. B RIS
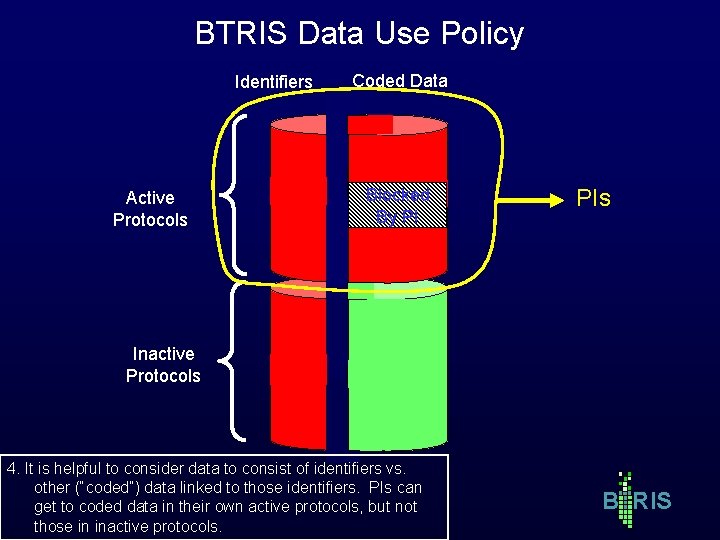
BTRIS Data Use Policy Identifiers Active Protocols Coded Data Blocked By PI PIs Inactive Protocols 4. It is helpful to consider data to consist of identifiers vs. other (“coded”) data linked to those identifiers. PIs can get to coded data in their own active protocols, but not those in inactive protocols. B RIS
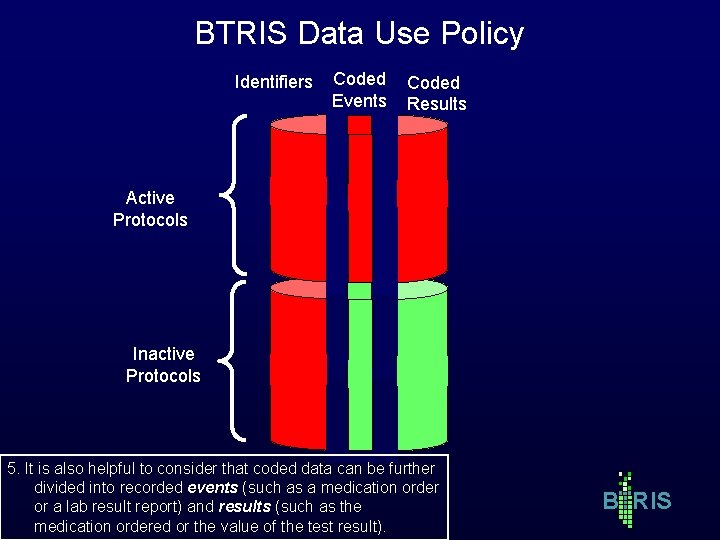
BTRIS Data Use Policy Identifiers Coded Events Coded Results Active Protocols Inactive Protocols 5. It is also helpful to consider that coded data can be further divided into recorded events (such as a medication order or a lab result report) and results (such as the medication ordered or the value of the test result). B RIS
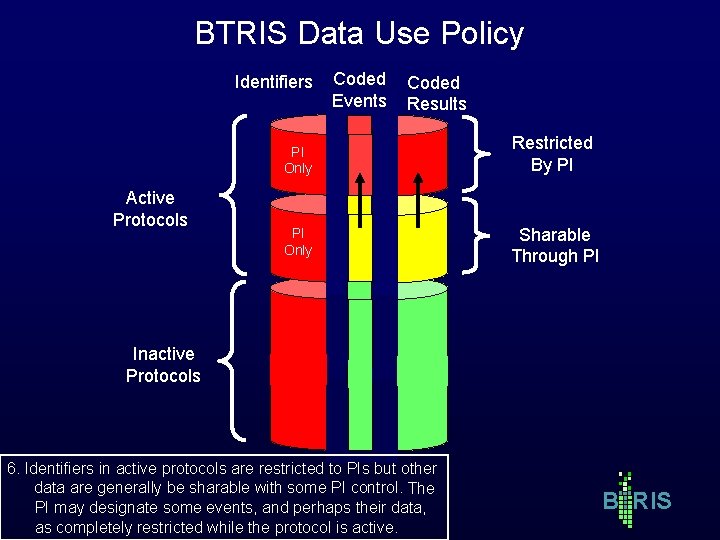
BTRIS Data Use Policy Identifiers Active Protocols Coded Events Coded Results PI Only Restricted By PI PI Only Sharable Through PI Inactive Protocols 6. Identifiers in active protocols are restricted to PIs but other data are generally be sharable with some PI control. The PI may designate some events, and perhaps their data, as completely restricted while the protocol is active. B RIS
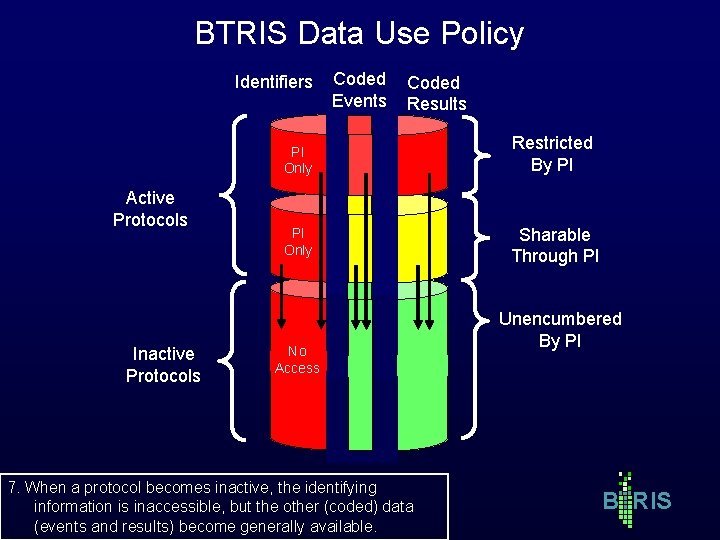
BTRIS Data Use Policy Identifiers Active Protocols Inactive Protocols Coded Events Coded Results PI Only Restricted By PI PI Only Sharable Through PI No Access 7. When a protocol becomes inactive, the identifying information is inaccessible, but the other (coded) data (events and results) become generally available. Unencumbered By PI B RIS
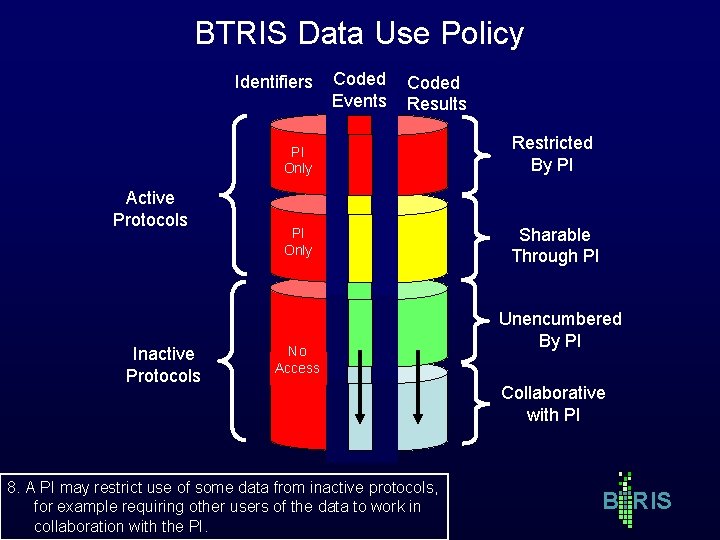
BTRIS Data Use Policy Identifiers Active Protocols Inactive Protocols Coded Events Coded Results PI Only Restricted By PI PI Only Sharable Through PI No Access 8. A PI may restrict use of some data from inactive protocols, for example requiring other users of the data to work in collaboration with the PI. Unencumbered By PI Collaborative with PI B RIS
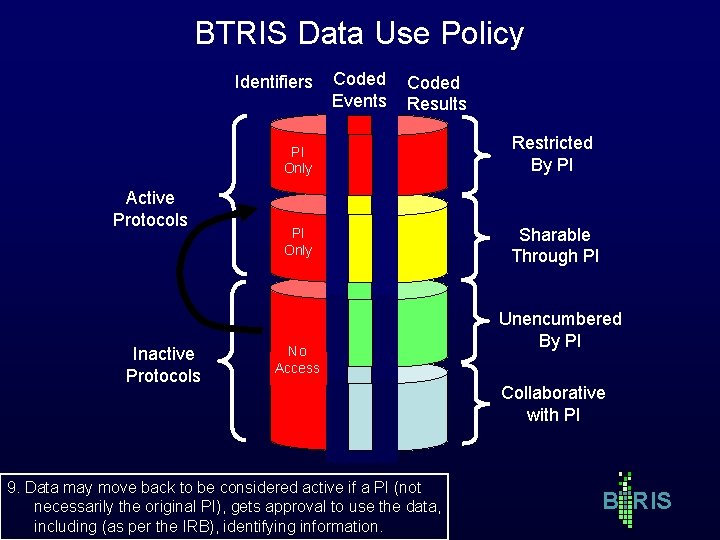
BTRIS Data Use Policy Identifiers Active Protocols Inactive Protocols Coded Events Coded Results PI Only Restricted By PI PI Only Sharable Through PI No Access 9. Data may move back to be considered active if a PI (not necessarily the original PI), gets approval to use the data, including (as per the IRB), identifying information. Unencumbered By PI Collaborative with PI B RIS
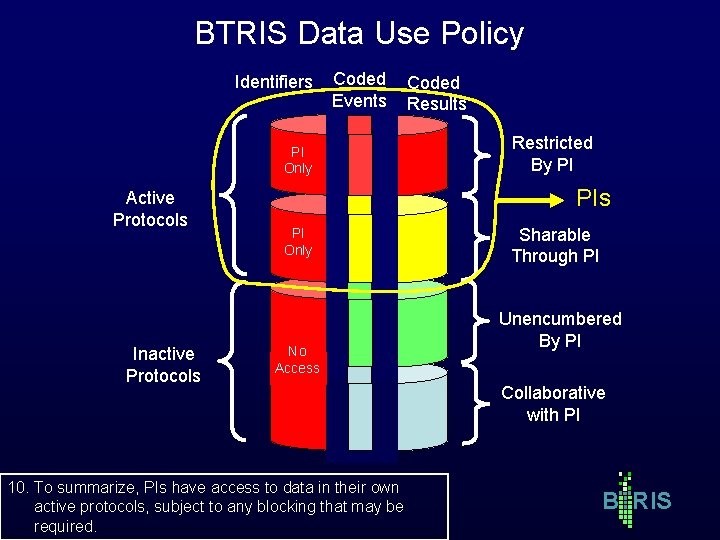
BTRIS Data Use Policy Identifiers Coded Events PI Only Active Protocols Inactive Protocols Coded Results Restricted By PI PIs PI Only No Access 10. To summarize, PIs have access to data in their own active protocols, subject to any blocking that may be required. Sharable Through PI Unencumbered By PI Collaborative with PI B RIS
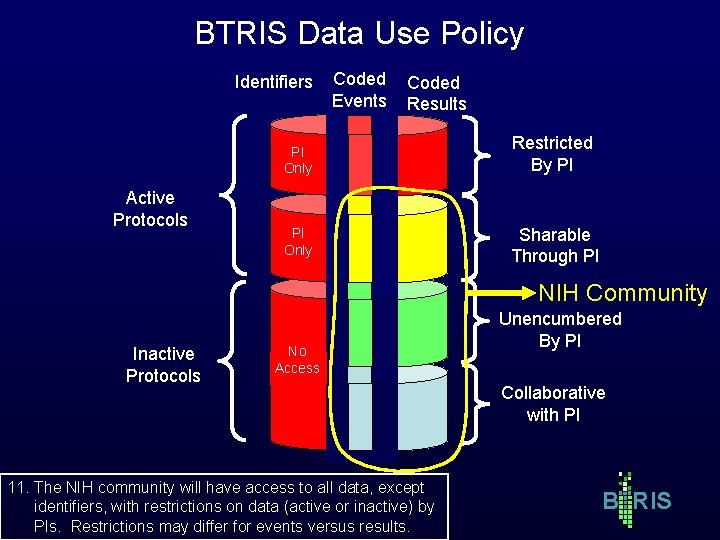
BTRIS Data Use Policy Identifiers Active Protocols Coded Events Coded Results PI Only Restricted By PI PI Only Sharable Through PI NIH Community Inactive Protocols No Access 11. The NIH community will have access to all data, except identifiers, with restrictions on data (active or inactive) by PIs. Restrictions may differ for events versus results. Unencumbered By PI Collaborative with PI B RIS

User Requirements • 2006 study of user requirements B RIS
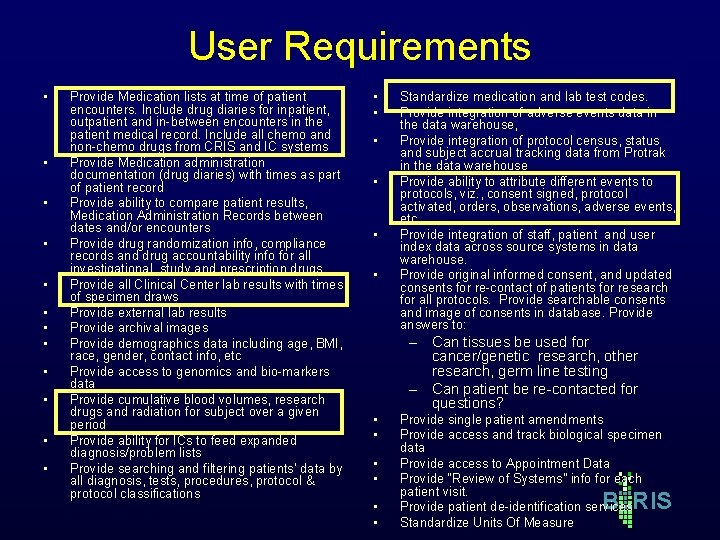
User Requirements • • • Provide Medication lists at time of patient encounters. Include drug diaries for inpatient, outpatient and in-between encounters in the patient medical record. Include all chemo and non-chemo drugs from CRIS and IC systems Provide Medication administration documentation (drug diaries) with times as part of patient record Provide ability to compare patient results, Medication Administration Records between dates and/or encounters Provide drug randomization info, compliance records and drug accountability info for all investigational, study and prescription drugs Provide all Clinical Center lab results with times of specimen draws Provide external lab results Provide archival images Provide demographics data including age, BMI, race, gender, contact info, etc Provide access to genomics and bio-markers data Provide cumulative blood volumes, research drugs and radiation for subject over a given period Provide ability for ICs to feed expanded diagnosis/problem lists Provide searching and filtering patients' data by all diagnosis, tests, procedures, protocol & protocol classifications • • • Standardize medication and lab test codes. Provide integration of adverse events data in the data warehouse, Provide integration of protocol census, status and subject accrual tracking data from Protrak in the data warehouse Provide ability to attribute different events to protocols, viz. , consent signed, protocol activated, orders, observations, adverse events, etc Provide integration of staff, patient and user index data across source systems in data warehouse. Provide original informed consent, and updated consents for re-contact of patients for research for all protocols. Provide searchable consents and image of consents in database. Provide answers to: – Can tissues be used for cancer/genetic research, other research, germ line testing – Can patient be re-contacted for questions? • • • Provide single patient amendments Provide access and track biological specimen data Provide access to Appointment Data Provide “Review of Systems” info for each patient visit. Provide patient de-identification services Standardize Units Of Measure B RIS

User Requirements • 2006 study of user requirements • Investigator User Group • Demos: – To user group – One-on-One – Town hall meeting B RIS
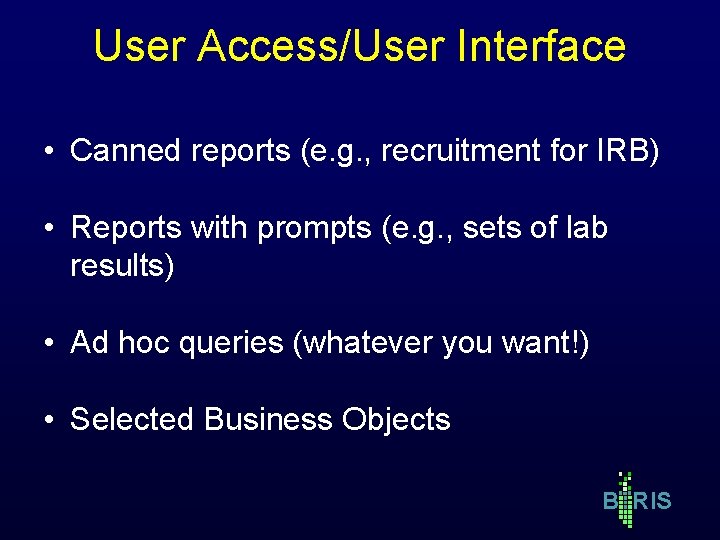
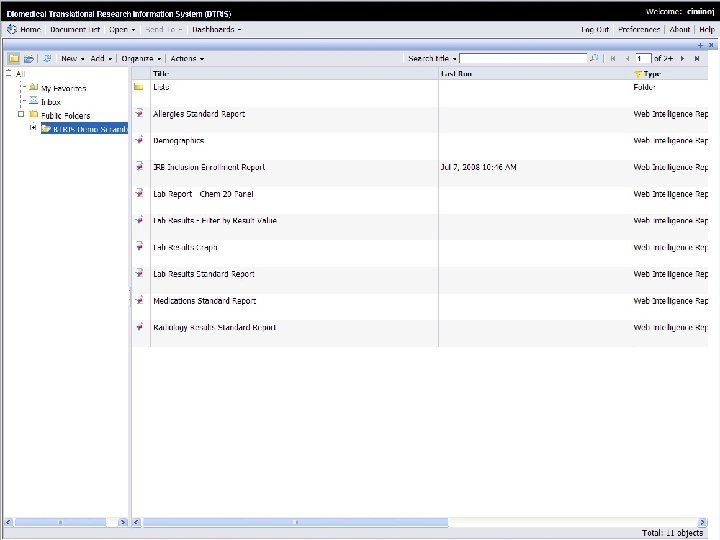
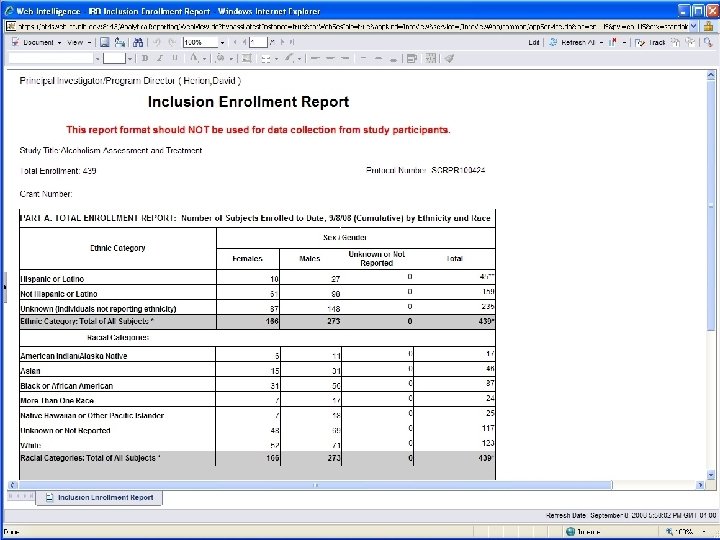
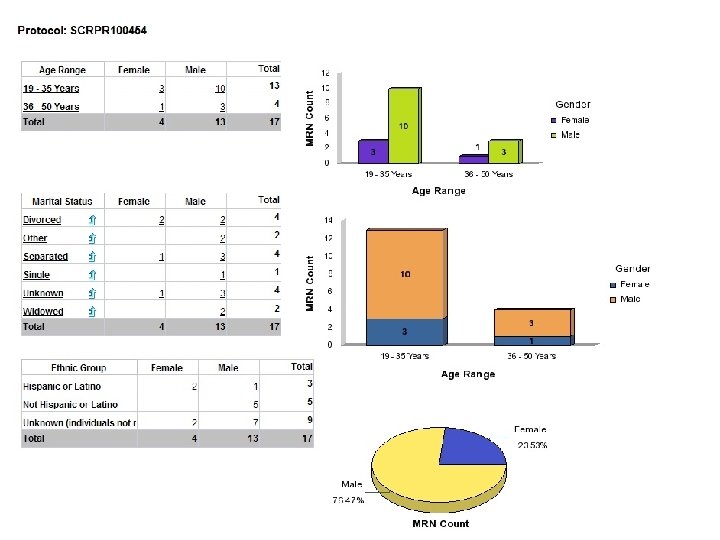
User Access/User Interface • Canned reports (e. g. , recruitment for IRB) • Reports with prompts (e. g. , sets of lab results) • Ad hoc queries (whatever you want!) • Selected Business Objects B RIS

Terminology-Based Queries • Hripcsak G, Allen B, Cimino JJ, Lee R. Access to Data: Comparing Access. Med to Query by Review. Journal of the American Medical Informatics Association; 1996; 3(4): 288 -299. B RIS

B RIS

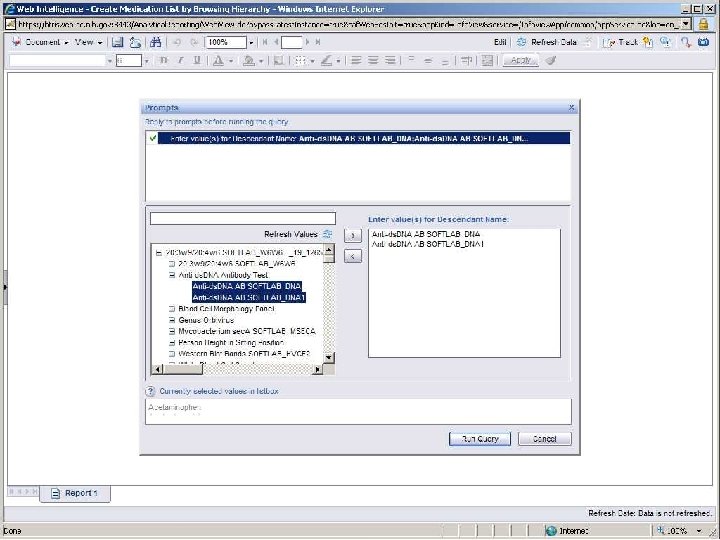
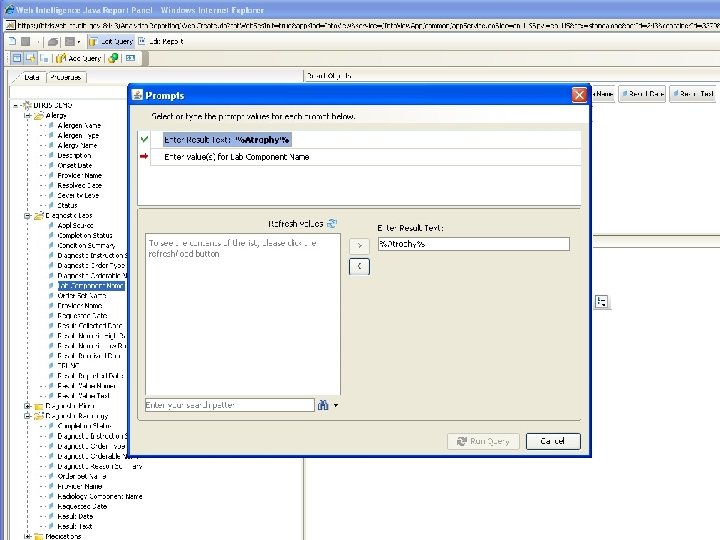
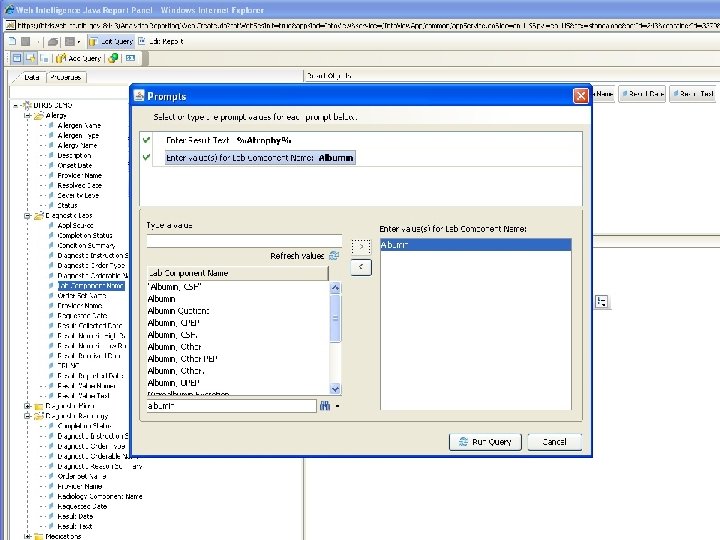
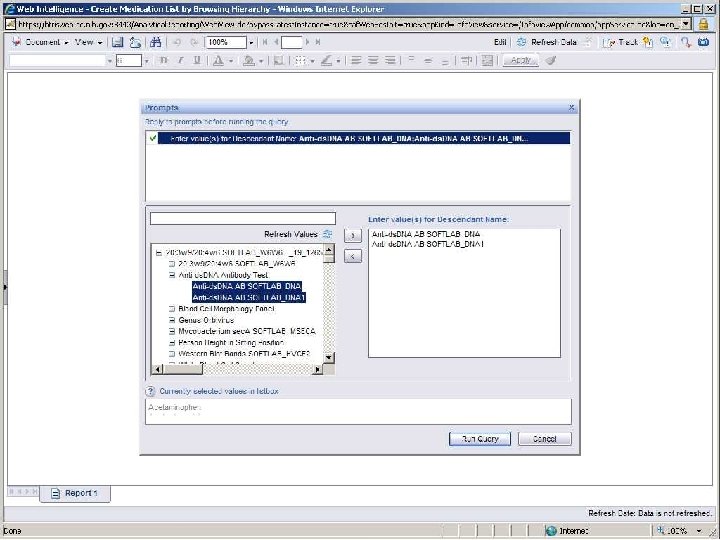
Terminology-Based Queries • Hripcsak G, Allen B, Cimino JJ, Lee R. Access to Data: Comparing Access. Med to Query by Review. Journal of the American Medical Informatics Association; 1996; 3(4): 288 -299 • Business Objects prompt with hierarchy B RIS
B RIS

Terminology-Based Queries • Hripcsak G, Allen B, Cimino JJ, Lee R. Access to Data: Comparing Access. Med to Query by Review. Journal of the American Medical Informatics Association; 1996; 3(4): 288 -299. • Business Objects prompt with hierarchy • Ancestor-descendant join with filtering B RIS
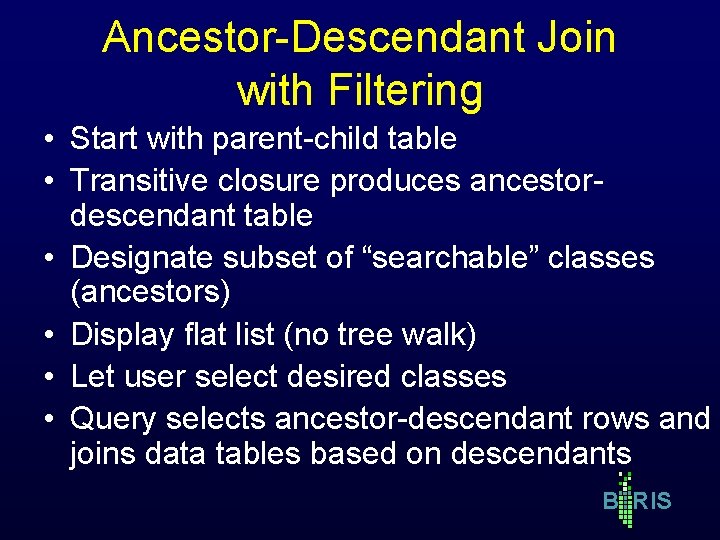
Ancestor-Descendant Join with Filtering • Start with parent-child table • Transitive closure produces ancestordescendant table • Designate subset of “searchable” classes (ancestors) • Display flat list (no tree walk) • Let user select desired classes • Query selects ancestor-descendant rows and joins data tables based on descendants B RIS
Status • Modeling source data – Clinical Research Information System – Clinical Data Repository – Crimson (NIAID) B RIS

B RIS
Status • Modeling source data – Clinical Research Information System – Clinical Data Repository – Crimson (NIAID) • Unified BTRIS data model B RIS

B RIS
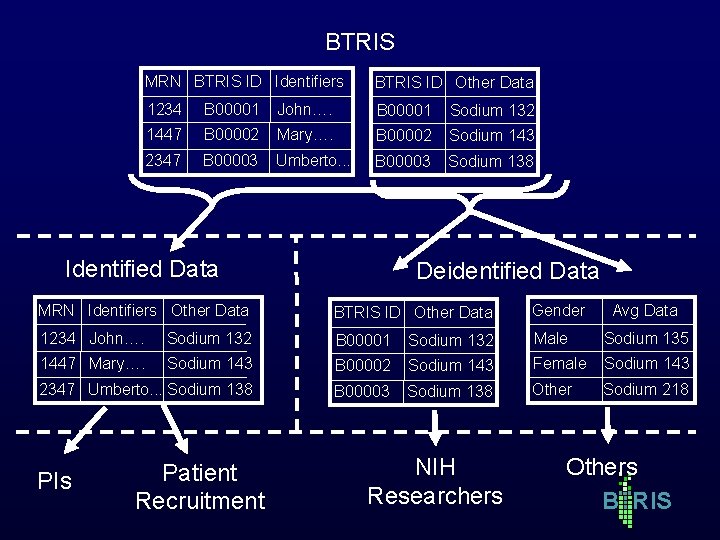
BTRIS MRN BTRIS ID Identifiers BTRIS ID Other Data 1234 B 00001 John…. B 00001 Sodium 132 1447 B 00002 Mary…. B 00002 Sodium 143 2347 B 00003 Umberto. . . B 00003 Sodium 138 Identified Data Deidentified Data MRN Identifiers Other Data BTRIS ID Other Data Gender 1234 John…. Sodium 132 B 00001 Sodium 132 Male Sodium 135 1447 Mary…. Sodium 143 B 00002 Sodium 143 Female Sodium 143 2347 Umberto. . . Sodium 138 B 00003 Sodium 138 Other Sodium 218 PIs Patient Recruitment NIH Researchers Avg Data Others B RIS
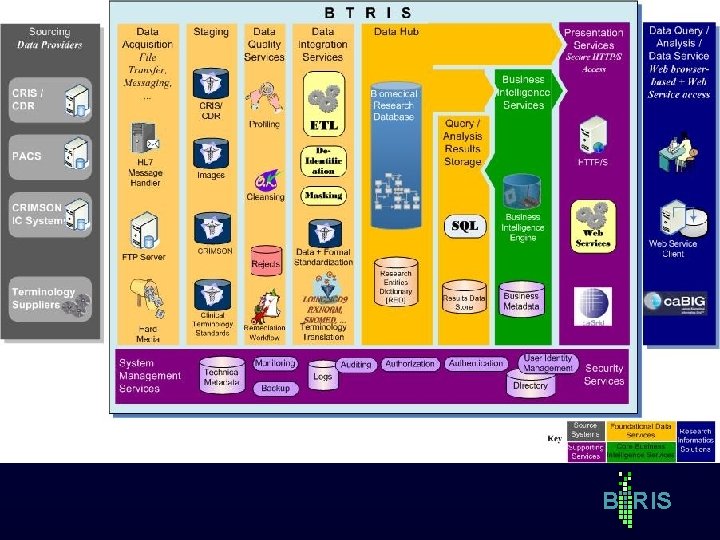
B RIS
Status • Modeling source data – Clinical Research Information System – Clinical Data Repository – Crimson (NIAID) • Unified BTRIS data model • Database versions – Specific for principal investigator – Scrambled data for general use • Business Objects demonstration B RIS
B RIS
B RIS
B RIS
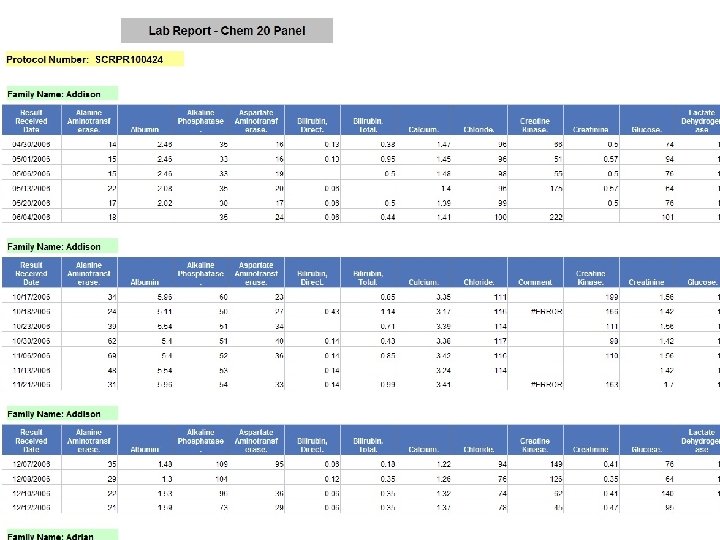
B RIS
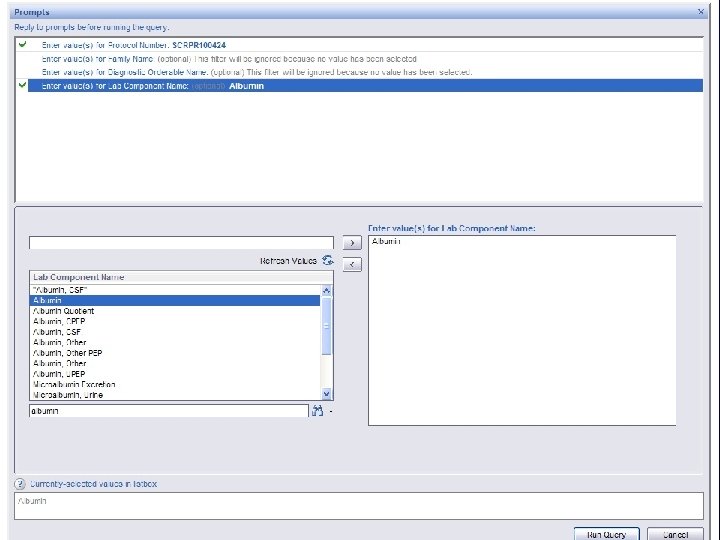
B RIS
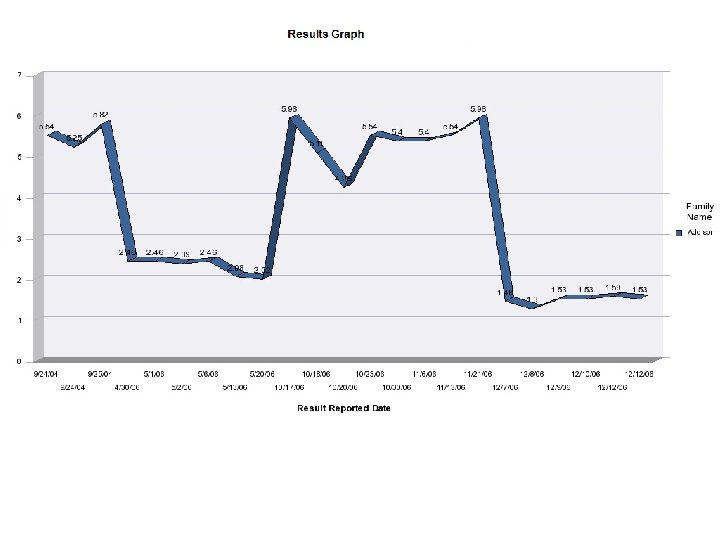
B RIS
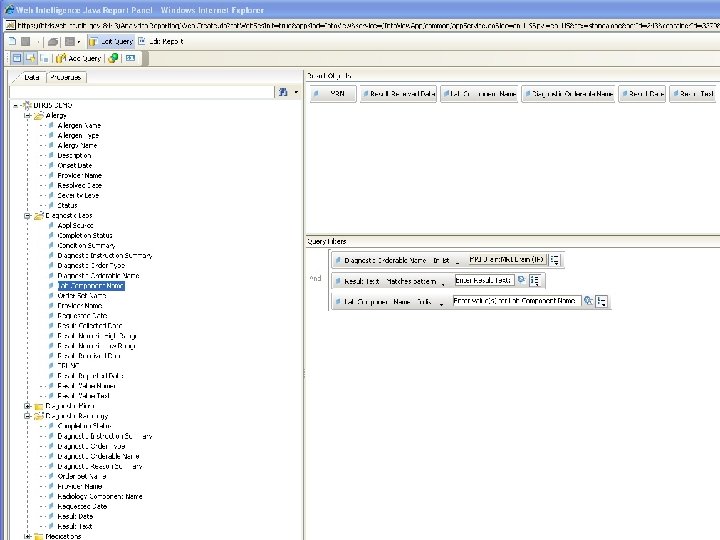
B RIS
B RIS
B RIS
B RIS
B RIS
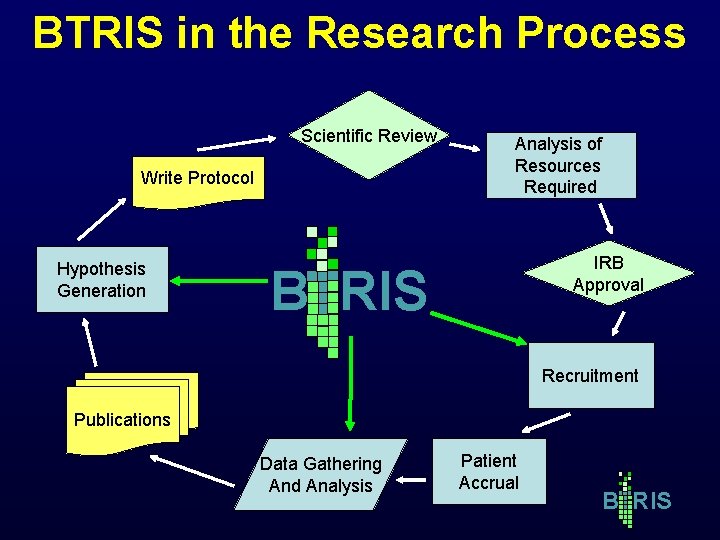
BTRIS in the Research Process Scientific Review Write Protocol Hypothesis Generation Analysis of Resources Required IRB Approval B RIS Recruitment Publications Data Gathering And Analysis Patient Accrual B RIS

Next Steps • Rebuild database in Oracle • Total historical data sets from CDR, CRIS, CRIMSON • Live interfaces with Lab, Radiology, CRIS and CRIMSON • Continue to collect user feedback • Develop report sets • BTRIS 1. 0 - July 2009 B RIS
More Issues • Patient ownership of de-identified data • If genomic data are included, can we truly de-identify? • Is protocol number a patient identifier? • How important is protocol attribution? • What data should not be shown to PIs? • What is the extent of data control by PIs? B RIS

Moving Forward • Despite centuries of clinical research, data are still fragmented • Sophisticated terminology is the key to reuse of disparate data • Policy issues are bigger than technical issues B RIS
BTRIS Will: • Be the preferred system to analyze NIH clinical and non-clinical data • Aggregate and standardize disparate and isolated data sets • Automate and streamline processes that are traditionally manual and cumbersome • Prioritize data sources and functionality based on needs of user community • Future of BTRIS: today the NIH, tomorrow B RIS the world?
btris. nih. gov B RIS
- Slides: 74