The BIG FAMILY of ORGANIC COMPOUNDS CARBON Forms

The BIG FAMILY of ORGANIC COMPOUNDS CARBON Forms All Organic Molecules Hydro. Carbon C-C Alcohols R-OH Ethers R-O-R’ O Esters R-CO-R O CELL Organic Acid R-COH R’ Lipides -Phospholipides – Amino acid Enzymes Saccharides –ect And MACROMOLECULES DNA –RNA –Proteins Polysaccharides, etc. Ammines R-N-R’’ O Aldehydes R-CH O Ketones R-C-R’ O
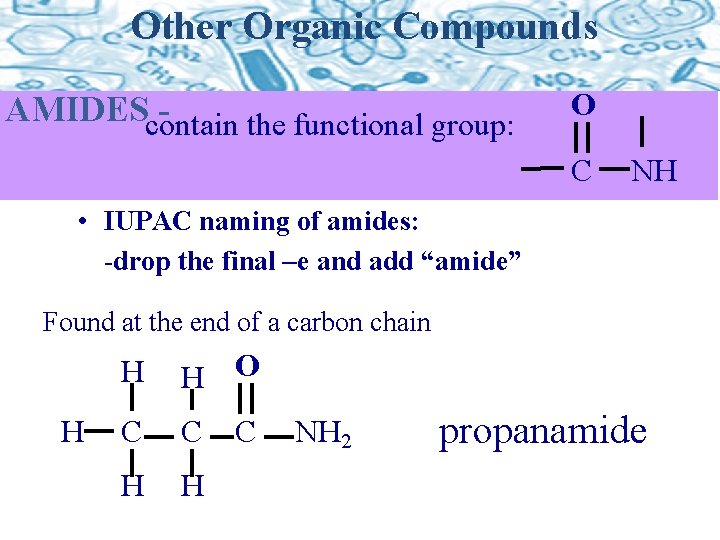
Other Organic Compounds AMIDEScontain the functional group: O C NH • IUPAC naming of amides: -drop the final –e and add “amide” Found at the end of a carbon chain H H H O C C H H C NH 2 propanamide
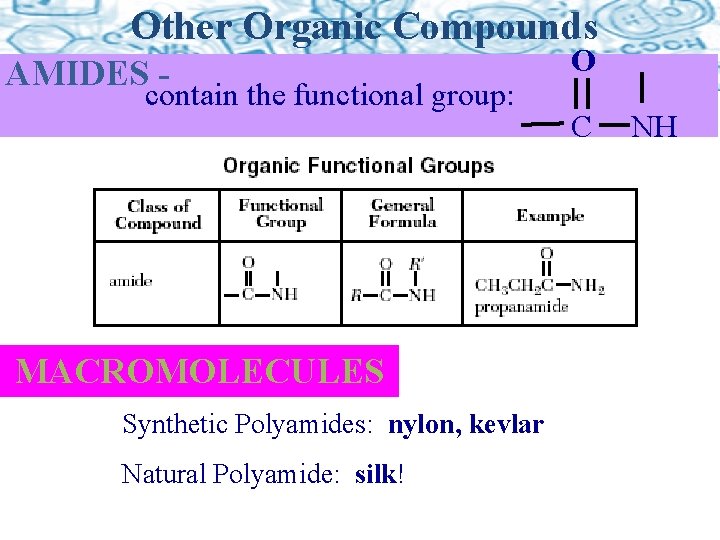
Other Organic Compounds AMIDES - contain the functional group: MACROMOLECULES Synthetic Polyamides: nylon, kevlar Natural Polyamide: silk! O C NH
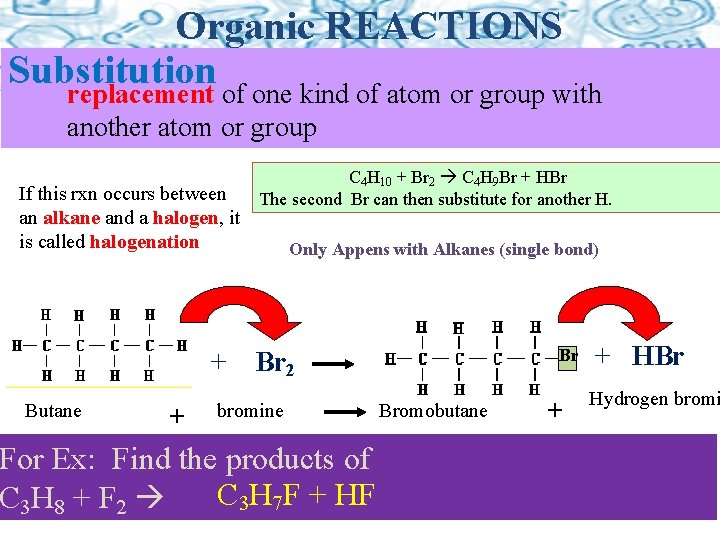
Organic REACTIONS Substitution replacement of one kind of atom or group with another atom or group If this rxn occurs between an alkane and a halogen, it is called halogenation + Butane + C 4 H 10 + Br 2 C 4 H 9 Br + HBr The second Br can then substitute for another H. Only Appens with Alkanes (single bond) Br Br 2 bromine For Ex: Find the products of C 3 H 7 F + HF C 3 H 8 + F 2 Bromobutane + + HBr Hydrogen bromi
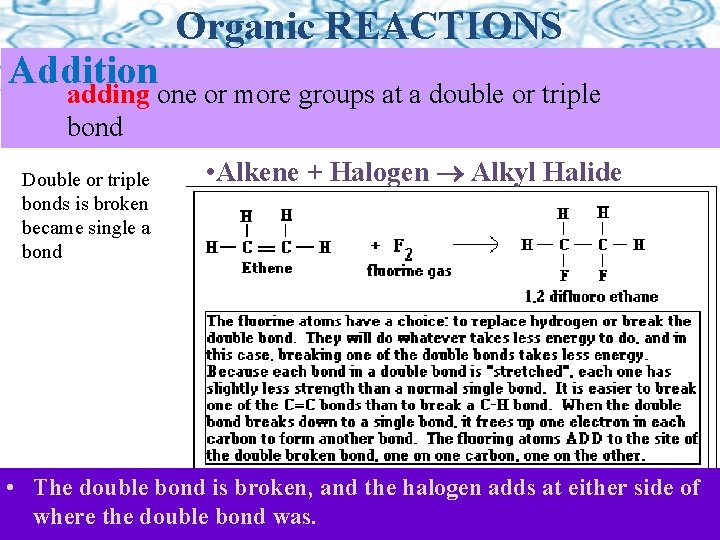
Addition Organic REACTIONS adding one or more groups at a double or triple bond Double or triple bonds is broken became single a bond • Alkene + Halogen Alkyl Halide Only Appens with Alkenes and Alkynes (double and triple bonds) • The double bond is broken, and the halogen adds at either side of where the double bond was.
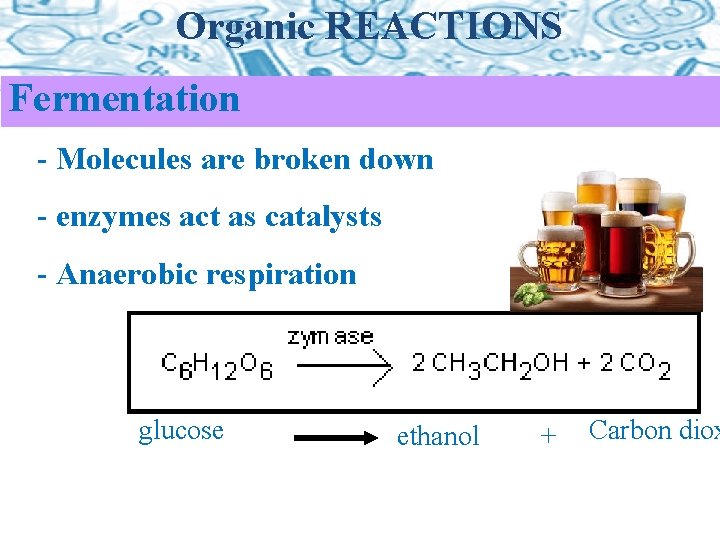
Organic REACTIONS Fermentation - Molecules are broken down - enzymes act as catalysts - Anaerobic respiration glucose ethanol + Carbon diox

Organic REACTIONS Esterification Organic Acid + Alcohol Ester + Water R-COOH R’-OH R-COOR’ -These are slow reversible reactions - similar to neutralization rxn in inorganic Acid + base = salt + water HCl + Na. OH = Na. Cl + H 2 O -H 2 O

Organic REACTIONS Saponification: Hydrolysis of fats The breaking of an ester to produce an organic acid plus an alcohol. Fat + water = organic acid + glycerol (glycerol ester) (soap) (alcohol) What is the reverse process of? Esterification Organic Acid + Alcohol Ester + Water

Organic REACTIONS Saponification: Hydrolysis of an ester in presence of a hot base (alkali) Glycerol ester + 3 Na. OH soap + glycerol Fat base alcohol soap
Other Organic Componds Polymers: are composed of many repeating units of monomers • Natural polymers starch – long chains of sugars proteins – long chains of amino acids cellulose – made of repeating units of sugar

Other Organic Componds Synthetic Polymers: man made polymers NYLON RAYON POLYESTER POLYETHYLENE SILICONE
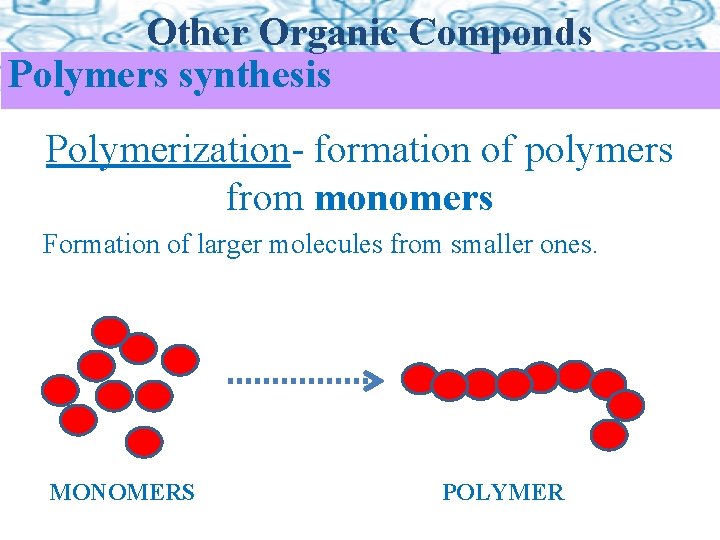
Other Organic Componds Polymers synthesis Polymerization- formation of polymers from monomers Formation of larger molecules from smaller ones. MONOMERS POLYMER
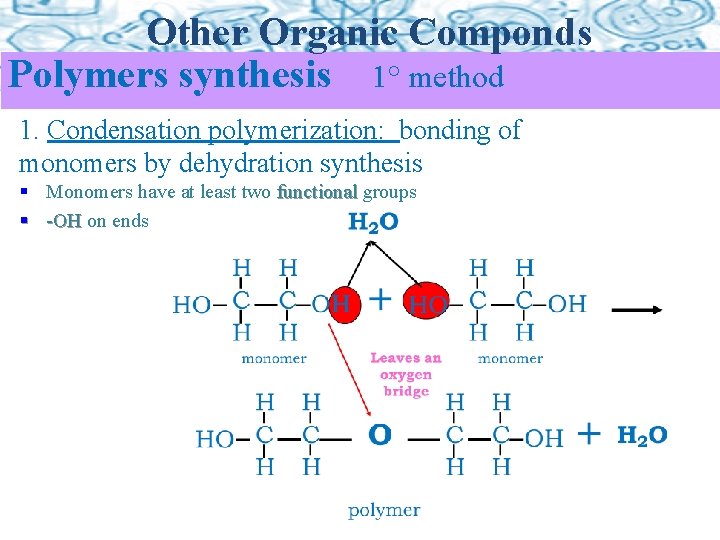
Other Organic Componds Polymers synthesis 1° method 1. Condensation polymerization: bonding of monomers by dehydration synthesis § Monomers have at least two functional groups § -OH on ends
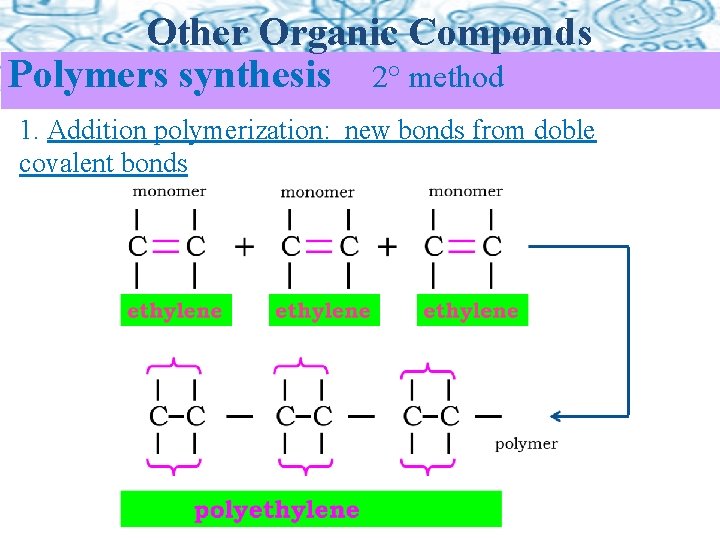
Other Organic Componds Polymers synthesis 2° method 1. Addition polymerization: new bonds from doble covalent bonds

ORGANIC CHEMISTRY Is LIFE • Prof. ssa Sonia Leta
- Slides: 15