The Battery Writing Oxidation and Reduction Reactions You

The Battery Writing Oxidation and Reduction Reactions You will need a partner, a periodic table and the Activity Series on pg. 357.
The Voltaic Cell = A Battery • Anode = negative/Oxidation = losing electrons • Cathode = positive/Reduction = gaining electrons • Voltage: the unit of a battery • The electron in the battery ALWAYS flows from negative to positive
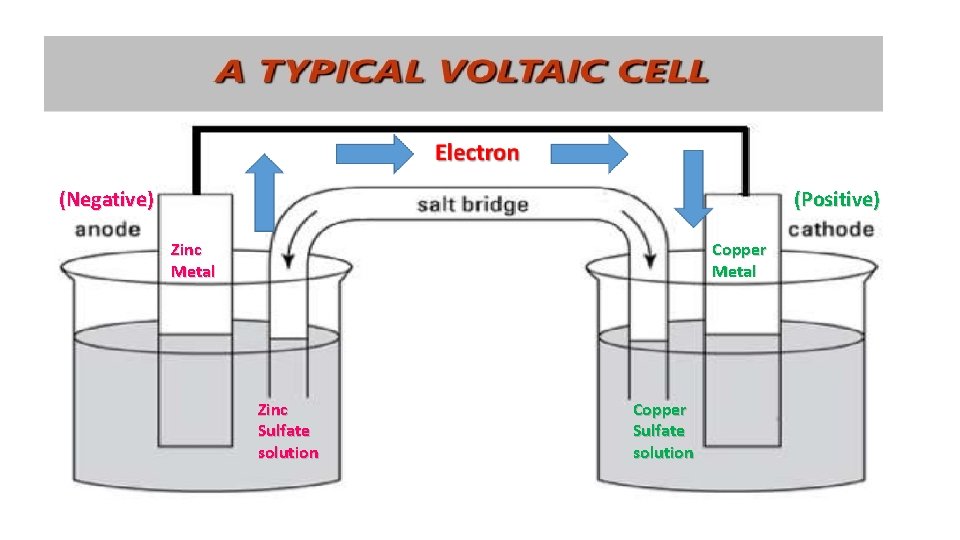
(Negative) (Positive) Copper Metal Zinc Sulfate solution Copper Sulfate solution

Using the Activity Series on pg. 357 • To determine which metal of the battery will be the negative (anode/Oxidation/losing electrons) and which metal of the battery will be the positive (cathode/Reduction/gaining electrons) you need to use the activity series. • The metal on the top of the activity series would be the negative (anode/Oxidation/losing electrons) and the metal on the bottom of the activity series will be the positive (cathode/Reduction/gaining electrons) you need to use the activity series.

Positive or Negative? The electron in the battery ALWAYS flows from negative to positive 1. 2. 3. 4. 5. Li ______ Mg ______ Fe _____ Ba _______ Au _____ Hg _______ Sn _____ Pb _______ K ______ Ni _______ Will this battery work? 1. _________ 2. _________ 3. _________ 4. _________ 5. _________

Positive or Negative? • 1. • 2. • 3. • 4. • 5. Li _Neg_ Fe _Pos_ Au _Pos_ Sn _Neg_ K _Neg_ Mg _Pos_ Ba _Neg_ Hg _Neg_ Pb _Pos_ Ni _Pos_
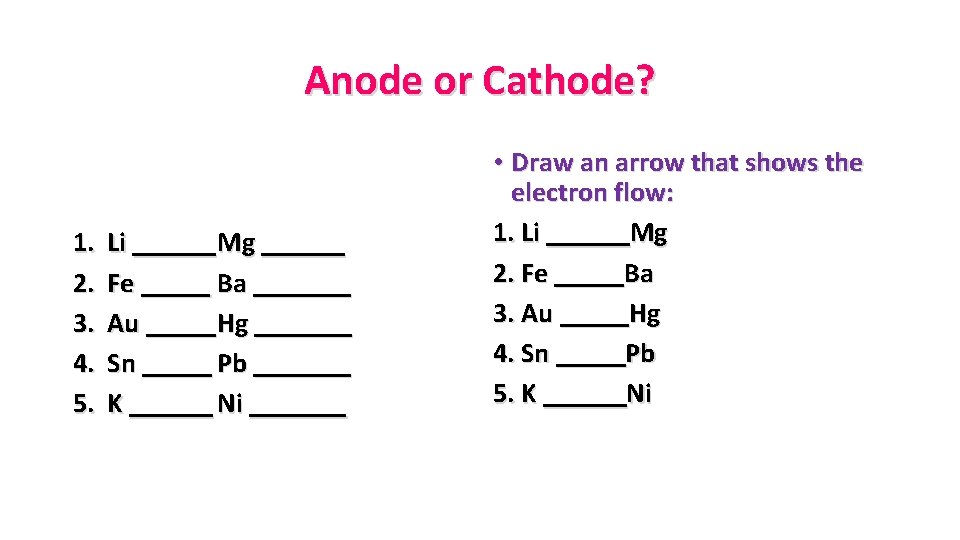
Anode or Cathode? 1. 2. 3. 4. 5. Li ______ Mg ______ Fe _____ Ba _______ Au _____ Hg _______ Sn _____ Pb _______ K ______ Ni _______ • Draw an arrow that shows the electron flow: 1. Li ______Mg 2. Fe _____Ba 3. Au _____Hg 4. Sn _____Pb 5. K ______Ni

Anode or Cathode? • 1. • 2. • 3. • 4. • 5. Li _Anode_ Fe _Cathode_ Au _Cathode_ Sn _Anode_ K _Anode_ Mg _Cathode_ Ba _Anode_ Hg _Anode_ Pb _Cathode_ Ni _Cathode_

Using the Activity Series on pg. 357 • The FARTHER APART the 2 metals are on the activity series, the BIGGER the voltage of the battery. • The CLOSER TOGETHER the 2 metals are on the activity series, the SMALLER the voltage of the battery. 1. Which battery would have the biggest voltage? How do you know? Li and Ba or Co and Au 2. Which battery would have the smallest voltage? How do you know? Fe and Ni OR Cu and Hg?
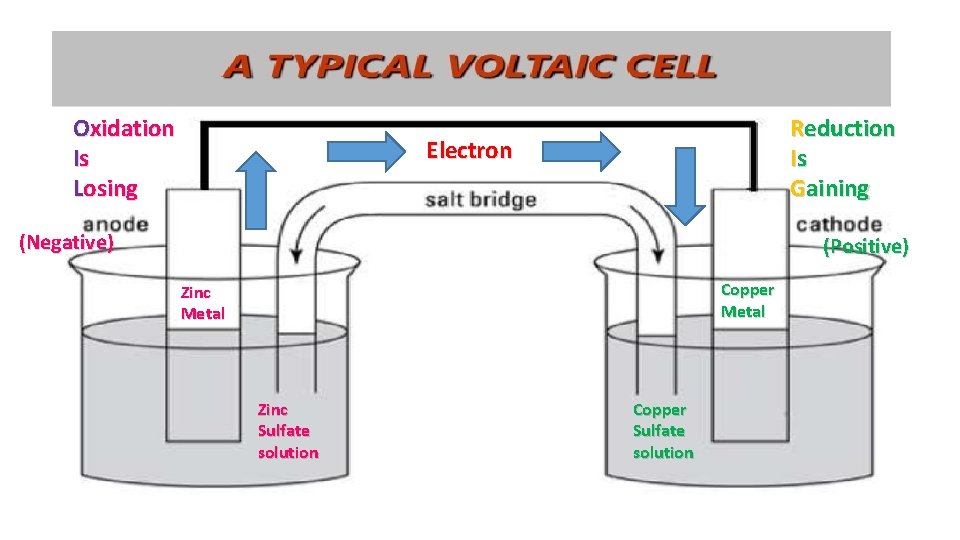
Oxidation Is Losing Reduction Is Gaining Electron (Negative) (Positive) Copper Metal Zinc Sulfate solution Copper Sulfate solution

OIL RIG • Oxidation Is Losing electrons 2+ • Ex. Mg + 2 electrons • Always starts out neutral Ends as a ion • Reduction Is Gaining electrons • Ex. S+2 + 2 electrons S • Always starts out as an ion Ends neutral
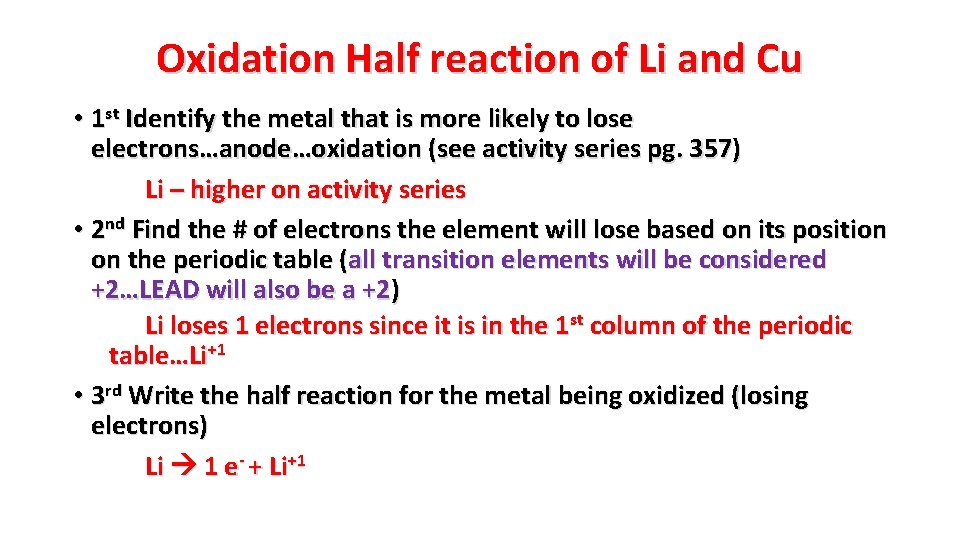
Oxidation Half reaction of Li and Cu • 1 st Identify the metal that is more likely to lose electrons…anode…oxidation (see activity series pg. 357) Li – higher on activity series • 2 nd Find the # of electrons the element will lose based on its position on the periodic table (all transition elements will be considered +2…LEAD will also be a +2) Li loses 1 electrons since it is in the 1 st column of the periodic table…Li+1 • 3 rd Write the half reaction for the metal being oxidized (losing electrons) Li 1 e- + Li+1
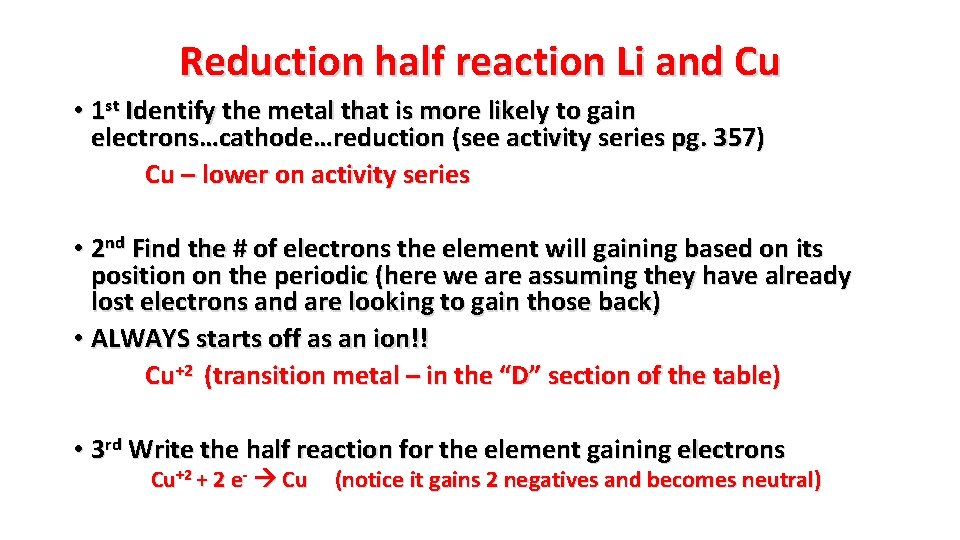
Reduction half reaction Li and Cu • 1 st Identify the metal that is more likely to gain electrons…cathode…reduction (see activity series pg. 357) Cu – lower on activity series • 2 nd Find the # of electrons the element will gaining based on its position on the periodic (here we are assuming they have already lost electrons and are looking to gain those back) • ALWAYS starts off as an ion!! Cu+2 (transition metal – in the “D” section of the table) • 3 rd Write the half reaction for the element gaining electrons Cu+2 + 2 e- Cu (notice it gains 2 negatives and becomes neutral)
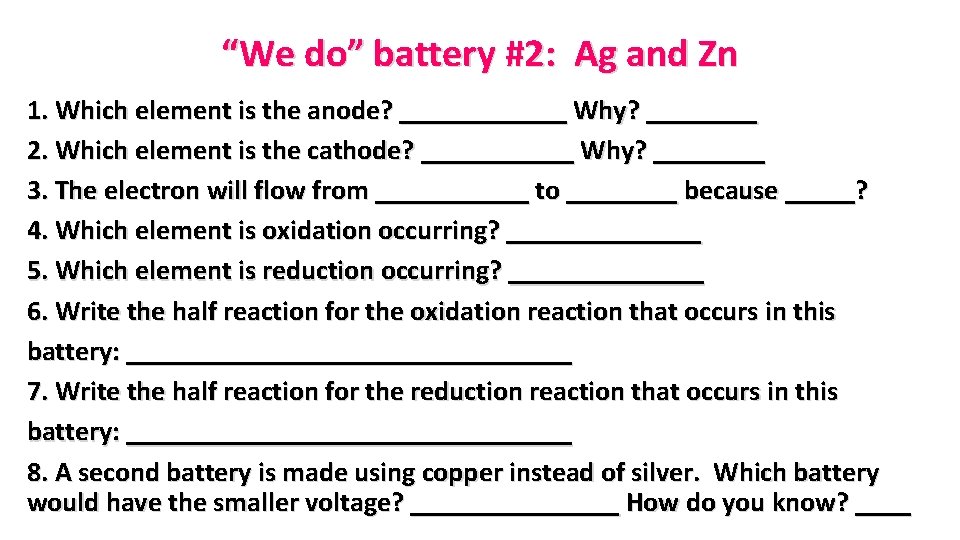
“We do” battery #2: Ag and Zn 1. Which element is the anode? ______ Why? ____ 2. Which element is the cathode? ______ Why? ____ 3. The electron will flow from ______ to ____ because _____? 4. Which element is oxidation occurring? _______ 5. Which element is reduction occurring? _______ 6. Write the half reaction for the oxidation reaction that occurs in this battery: ________________ 7. Write the half reaction for the reduction reaction that occurs in this battery: ________________ 8. A second battery is made using copper instead of silver. Which battery would have the smaller voltage? ________ How do you know? ____
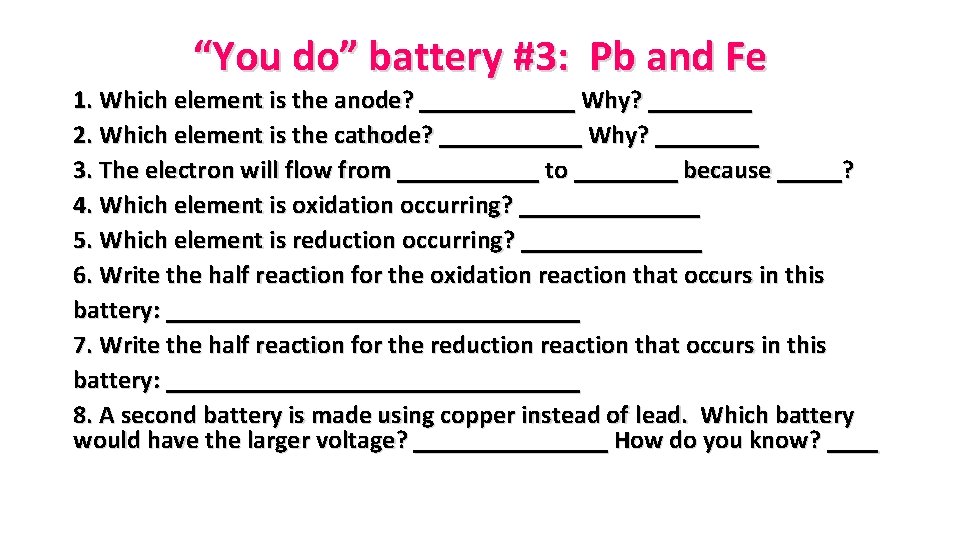
“You do” battery #3: Pb and Fe 1. Which element is the anode? ______ Why? ____ 2. Which element is the cathode? ______ Why? ____ 3. The electron will flow from ______ to ____ because _____? 4. Which element is oxidation occurring? _______ 5. Which element is reduction occurring? _______ 6. Write the half reaction for the oxidation reaction that occurs in this battery: ________________ 7. Write the half reaction for the reduction reaction that occurs in this battery: ________________ 8. A second battery is made using copper instead of lead. Which battery would have the larger voltage? ________ How do you know? ____
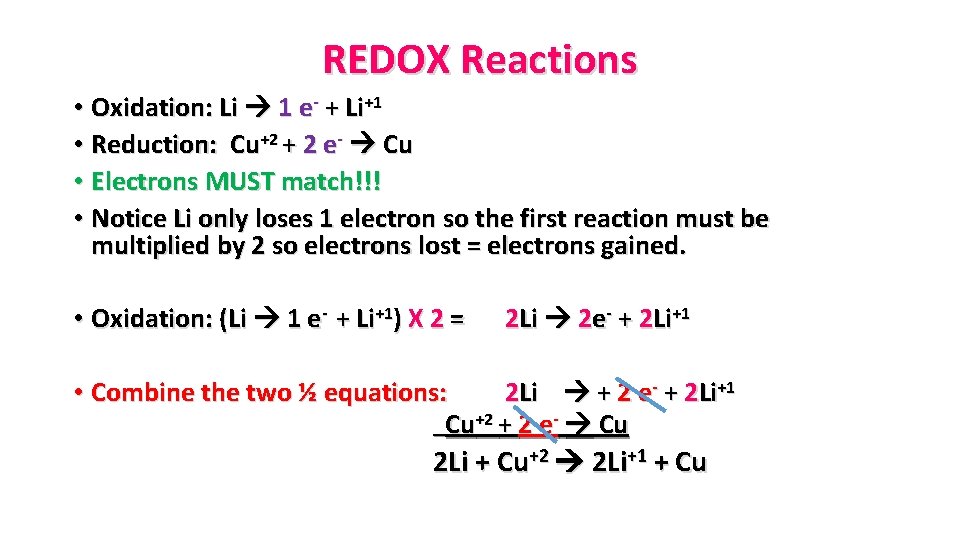
REDOX Reactions • Oxidation: Li 1 e- + Li+1 • Reduction: Cu+2 + 2 e- Cu • Electrons MUST match!!! • Notice Li only loses 1 electron so the first reaction must be multiplied by 2 so electrons lost = electrons gained. • Oxidation: (Li 1 e- + Li+1) X 2 = 2 Li 2 e- + 2 Li+1 • Combine the two ½ equations: 2 Li + 2 e- + 2 Li+1 Cu+2 + 2 e- Cu 2 Li + Cu+2 2 Li+1 + Cu
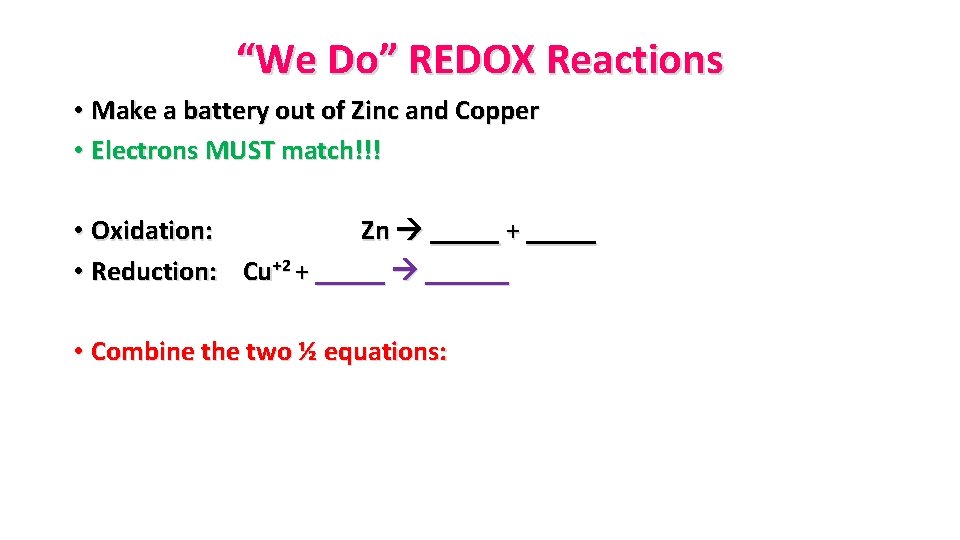
“We Do” REDOX Reactions • Make a battery out of Zinc and Copper • Electrons MUST match!!! • Oxidation: Zn _____ + _____ • Reduction: Cu+2 + ______ • Combine the two ½ equations:

“You Do” REDOX Reactions • Make a battery out of Potassium and Tin • Electrons MUST match!!! • Oxidation: • Reduction: • Combine the two ½ equations:
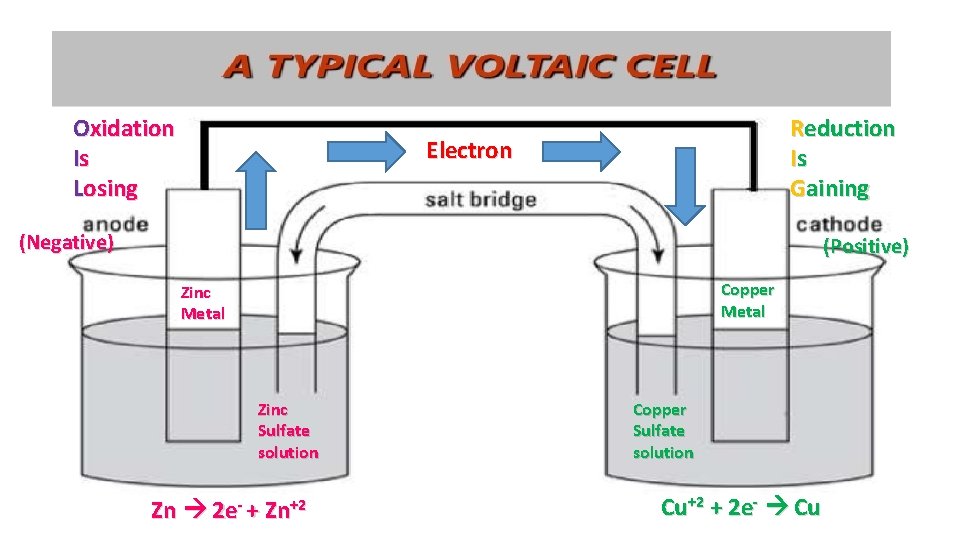
Oxidation Is Losing Reduction Is Gaining Electron (Negative) (Positive) Copper Metal Zinc Sulfate solution Zn 2 e- + Zn+2 Copper Sulfate solution Cu+2 + 2 e- Cu
- Slides: 19