The Basics of Classifying Matter Matter can be
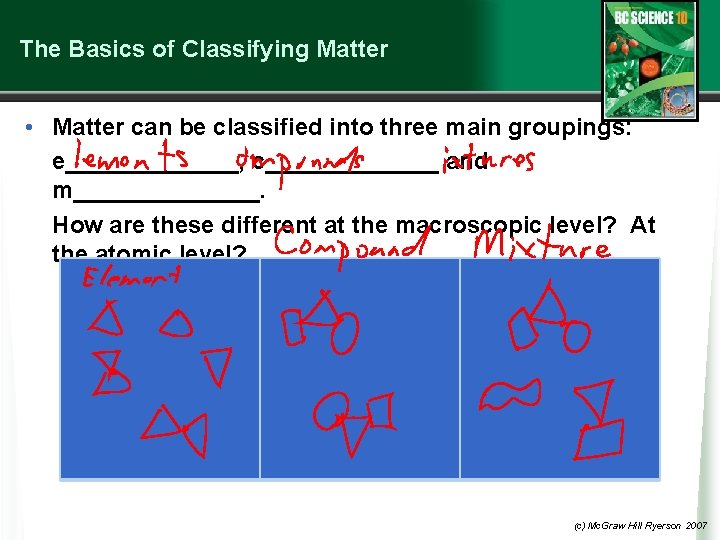
The Basics of Classifying Matter • Matter can be classified into three main groupings: e_______, c_______ and m_______. How are these different at the macroscopic level? At the atomic level? (c) Mc. Graw Hill Ryerson 2007
4. 1 Atomic Theory and Bonding • An atom is the smallest particle of an element that still has the properties of that element w 50 million atoms, lined up end to end = 1 cm w An atom = proton(s) + neutron(s) + electron(s) • Atoms join together to form compounds. w A compound is a pure substance that is composed of two or more atoms combined in a specific way. w Oxygen and hydrogen are atoms/elements; H 2 O is a compound. • A chemical change occurs when the arrangement of See pages 168 - 169 atoms in compounds changes to form new (c) Mc. Graw Hill Ryerson 2007
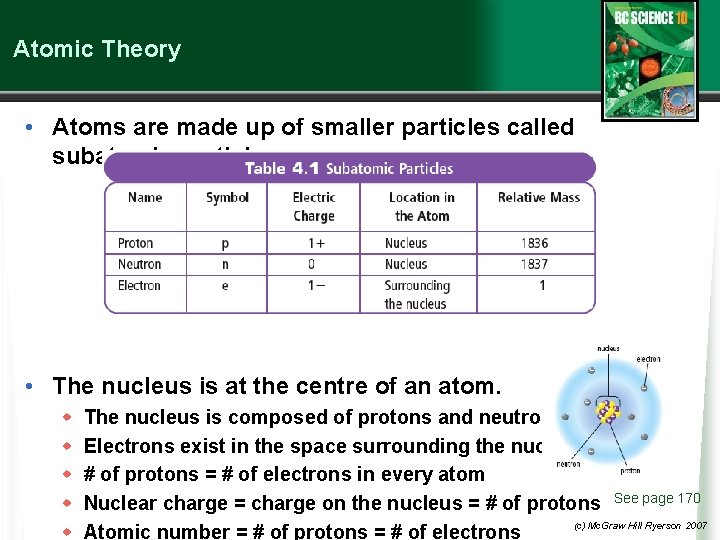
Atomic Theory • Atoms are made up of smaller particles called subatomic particles. • The nucleus is at the centre of an atom. w w w The nucleus is composed of protons and neutrons. Electrons exist in the space surrounding the nucleus. # of protons = # of electrons in every atom Nuclear charge = charge on the nucleus = # of protons See page 170 (c) Mc. Graw Hill Ryerson 2007 Atomic number = # of protons = # of electrons
Organization of the Periodic Table • In the periodic table elements are listed in order by their atomic number. w Metals are on the left (the transition metals range from group 3 to group 12), non-metals are on the right, and the metalloids form a “staircase” toward the right side. w Rows of elements (across) are called periods. § All elements in a period have their electrons in the same general area around their nucleus. w Columns of elements are called groups, or families. § All elements in a family have similar properties and bond with other elements in similar ways. § Group 1 = alkali metals § Group 2 = alkaline earth metals § Group 17 = the halogens § Group 18 = noble gases See page 171 (c) Mc. Graw Hill Ryerson 2007
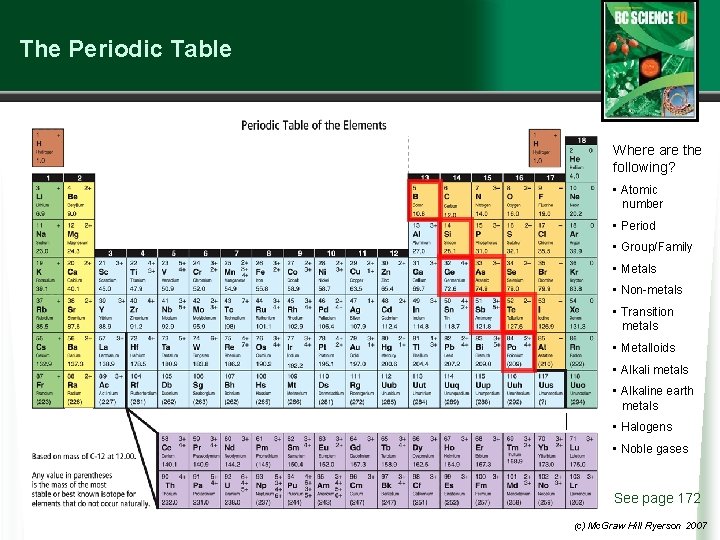
The Periodic Table INCREASING REACTIVITY Where are the following? • Atomic number • Period • Group/Family • Metals • Non-metals • Transition metals • Metalloids • Alkali metals • Alkaline earth metals • Halogens • Noble gases See page 172 (c) Mc. Graw Hill Ryerson 2007

A Periodic Table Task Research some facts about each of the following parts of the periodic table and prepare a short presentation for the class. Include trends in physical and chemical properties as well as some uses in everyday life. Present in Powerpoint form or as a printed summary. Transition elements Groups 1 and 2 Groups 13 -15 Groups 16 -17 Group 18 Lanthanides Actinides Metalloids (c) Mc. Graw Hill Ryerson 2007

Tomorrow’s Presentation This is a small research task, so does not count a huge amount. Contents: 5 (Some physical and chemical properties of the group/block/period, highlighting important aspects) Presentation: 5 (Interesting, clear) Let’s learn more about the building blocks of all matter, the elements. They are like the bricks of a great building, like the 26 letters of the alphabet that allow for the creation of millions of words, like…) (c) Mc. Graw Hill Ryerson 2007

Back to the atoms… 1. Atoms have protons and neutrons in the nucleus. Their numbers are roughly equal for smaller atoms. The numbers do not depend on each other. 2. In a neutral atom, the number of _______ equals the number of _________. 3. Atoms can only gain or lose ________. Why? 4. If an atom has more ______ than protons, it is a ______________. If fewer _______ than _____, then it is a ____________. 5. What determines whether _______ are gained or lost? (c) Mc. Graw Hill Ryerson 2007
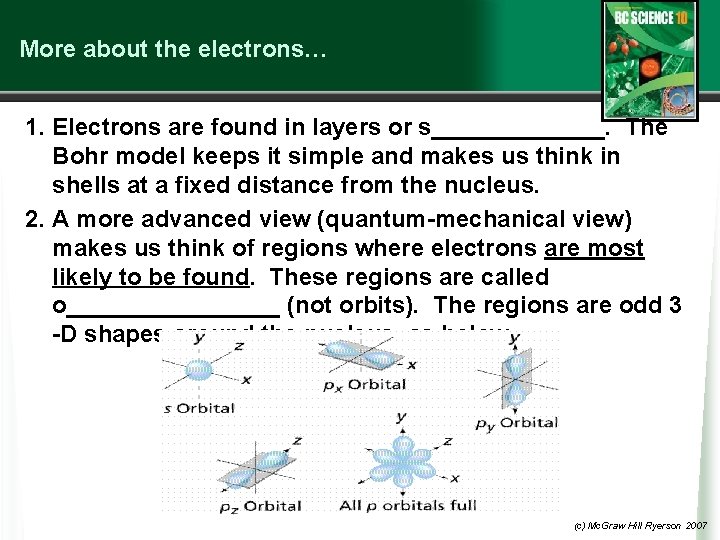
More about the electrons… 1. Electrons are found in layers or s_______. The Bohr model keeps it simple and makes us think in shells at a fixed distance from the nucleus. 2. A more advanced view (quantum-mechanical view) makes us think of regions where electrons are most likely to be found. These regions are called o________ (not orbits). The regions are odd 3 -D shapes around the nucleus, as below. (c) Mc. Graw Hill Ryerson 2007
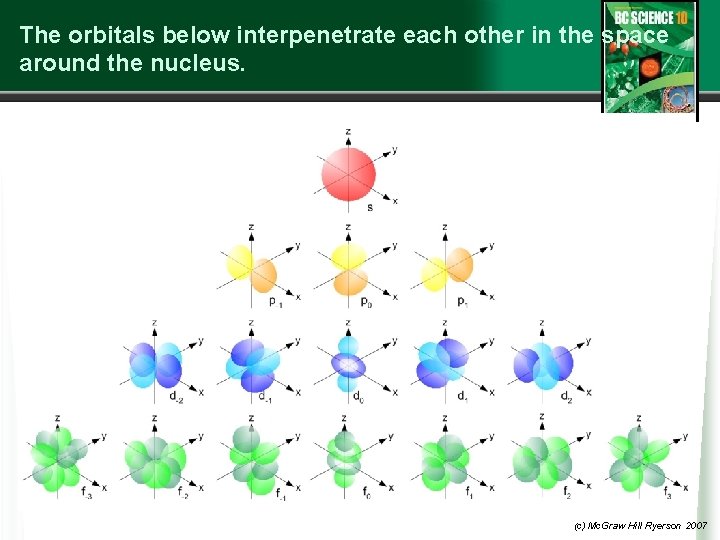
The orbitals below interpenetrate each other in the space around the nucleus. (c) Mc. Graw Hill Ryerson 2007
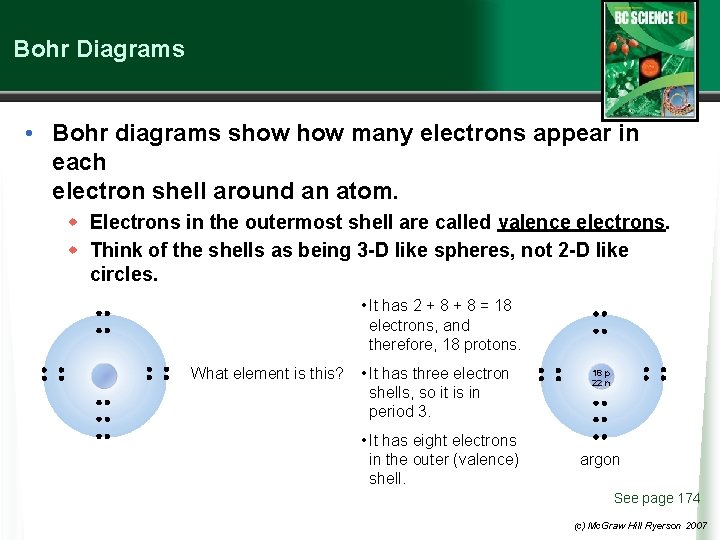
Bohr Diagrams • Bohr diagrams show many electrons appear in each electron shell around an atom. w Electrons in the outermost shell are called valence electrons. w Think of the shells as being 3 -D like spheres, not 2 -D like circles. • It has 2 + 8 = 18 electrons, and therefore, 18 protons. What element is this? • It has three electron shells, so it is in period 3. • It has eight electrons in the outer (valence) shell. 18 p 22 n argon See page 174 (c) Mc. Graw Hill Ryerson 2007
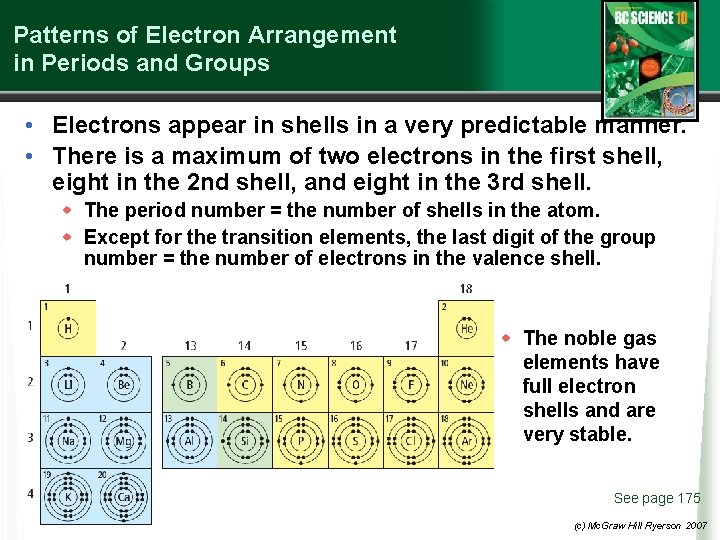
Patterns of Electron Arrangement in Periods and Groups • Electrons appear in shells in a very predictable manner. • There is a maximum of two electrons in the first shell, eight in the 2 nd shell, and eight in the 3 rd shell. w The period number = the number of shells in the atom. w Except for the transition elements, the last digit of the group number = the number of electrons in the valence shell. w The noble gas elements have full electron shells and are very stable. See page 175 (c) Mc. Graw Hill Ryerson 2007
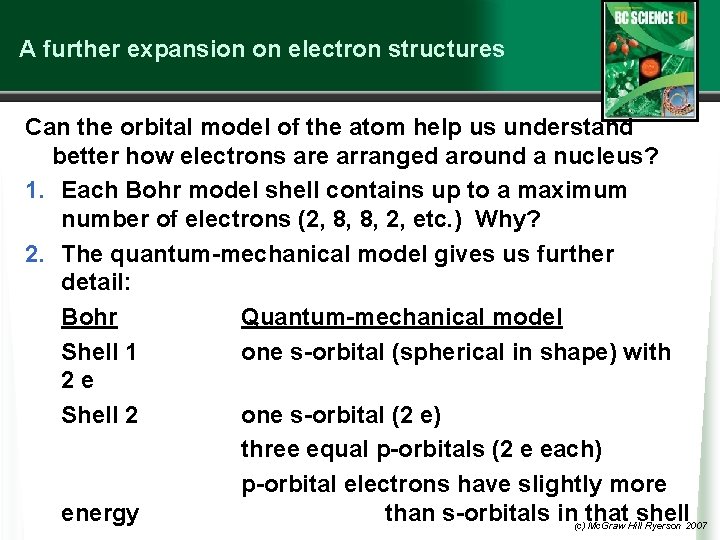
A further expansion on electron structures Can the orbital model of the atom help us understand better how electrons are arranged around a nucleus? 1. Each Bohr model shell contains up to a maximum number of electrons (2, 8, 8, 2, etc. ) Why? 2. The quantum-mechanical model gives us further detail: Bohr Quantum-mechanical model Shell 1 one s-orbital (spherical in shape) with 2 e Shell 2 one s-orbital (2 e) three equal p-orbitals (2 e each) p-orbital electrons have slightly more energy than s-orbitals in(c)that shell Mc. Graw Hill Ryerson 2007

Bohr Shell 3 QM model s-orbital with 2 e three p-orbitals with 2 e each Shell 4 s-orbital with 2 e Beyond this there is further filling of the 3 rd shell and 4 th shell but we will not go beyond 20 electrons. The diagram on the next slide helps us put this together. (c) Mc. Graw Hill Ryerson 2007
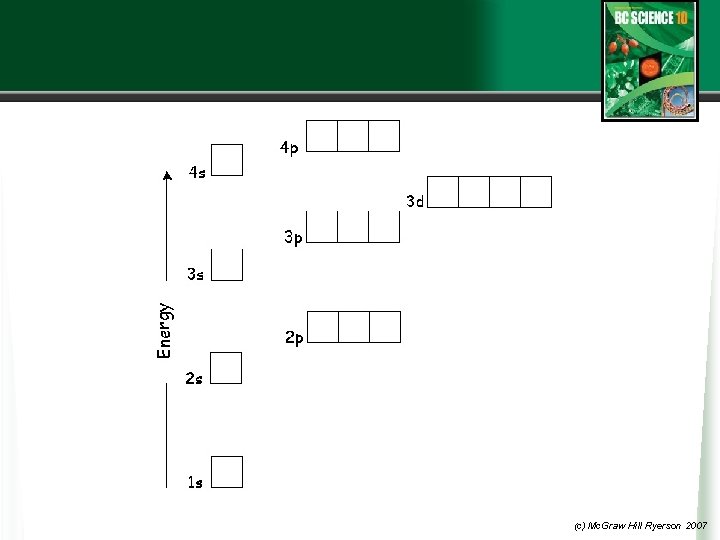
(c) Mc. Graw Hill Ryerson 2007
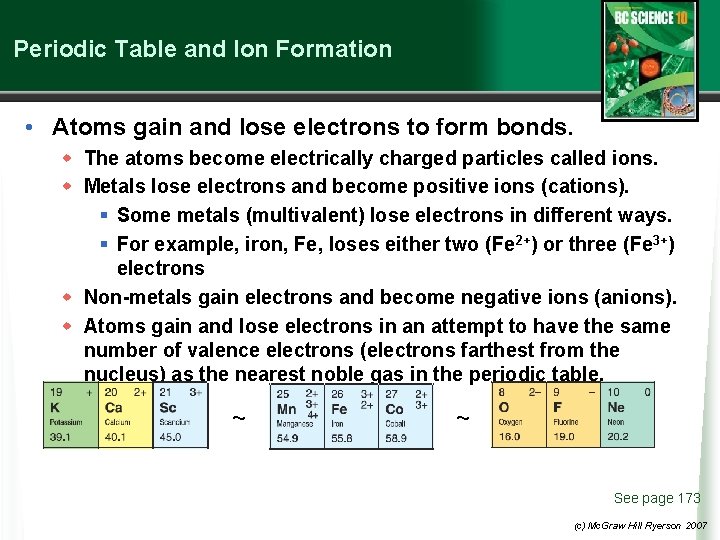
Periodic Table and Ion Formation • Atoms gain and lose electrons to form bonds. w The atoms become electrically charged particles called ions. w Metals lose electrons and become positive ions (cations). § Some metals (multivalent) lose electrons in different ways. § For example, iron, Fe, loses either two (Fe 2+) or three (Fe 3+) electrons w Non-metals gain electrons and become negative ions (anions). w Atoms gain and lose electrons in an attempt to have the same number of valence electrons (electrons farthest from the nucleus) as the nearest noble gas in the periodic table. ~ ~ See page 173 (c) Mc. Graw Hill Ryerson 2007

Forming Compounds • When two atoms get close together, their valence electrons interact. w If the valence electrons can combine to form a low-energy bond, a compound is formed. w Each atom in the compound attempts to have the stable number of valence electrons as the nearest noble gas. w Metals may lose electrons and non-metals may gain electrons (ionic bond), or atoms may share electrons (covalent bond). • Ionic bonds form when electrons are transferred from positive ions to negative ions. • Covalent bonds form when electrons are shared between two non-metals. See pages 176 - 177 w Electrons stay with their atom but overlap with other shells. (c) Mc. Graw Hill Ryerson 2007
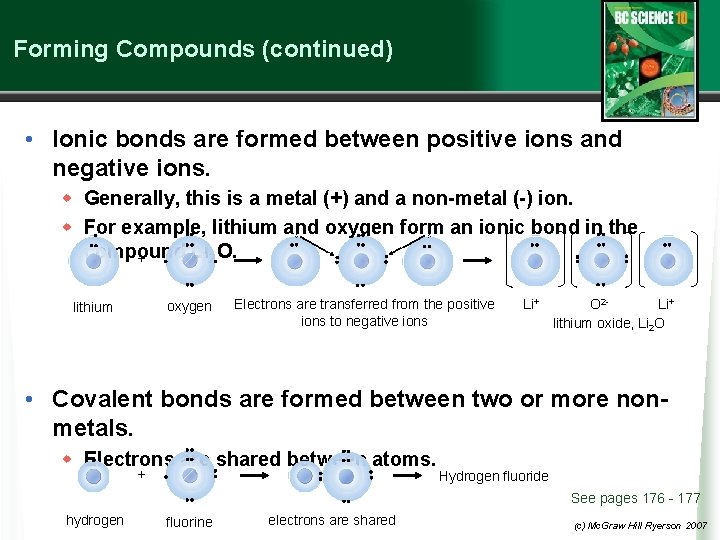
Forming Compounds (continued) • Ionic bonds are formed between positive ions and negative ions. w Generally, this is a metal (+) and a non-metal (-) ion. w For example, lithium and oxygen form an ionic bond in the compound Li 2 O. + oxygen lithium Electrons are transferred from the positive ions to negative ions Li+ O 2 Li+ lithium oxide, Li 2 O • Covalent bonds are formed between two or more nonmetals. w Electrons are shared between atoms. + Hydrogen fluoride See pages 176 - 177 hydrogen fluorine electrons are shared (c) Mc. Graw Hill Ryerson 2007
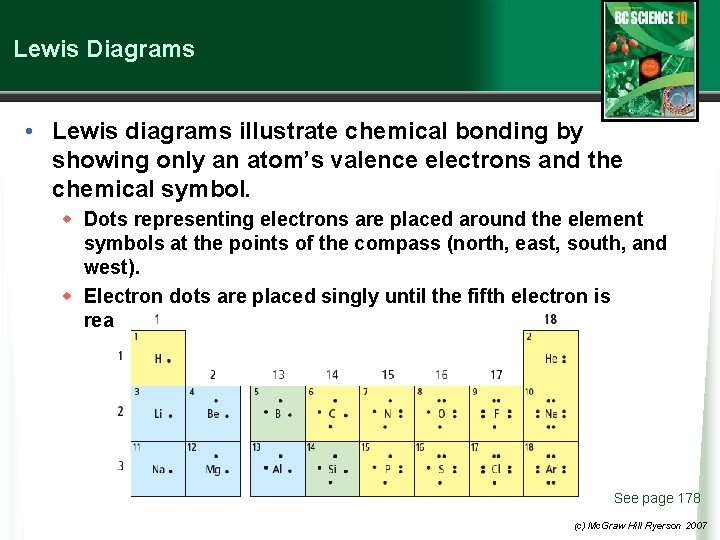
Lewis Diagrams • Lewis diagrams illustrate chemical bonding by showing only an atom’s valence electrons and the chemical symbol. w Dots representing electrons are placed around the element symbols at the points of the compass (north, east, south, and west). w Electron dots are placed singly until the fifth electron is reached then they are paired. See page 178 (c) Mc. Graw Hill Ryerson 2007
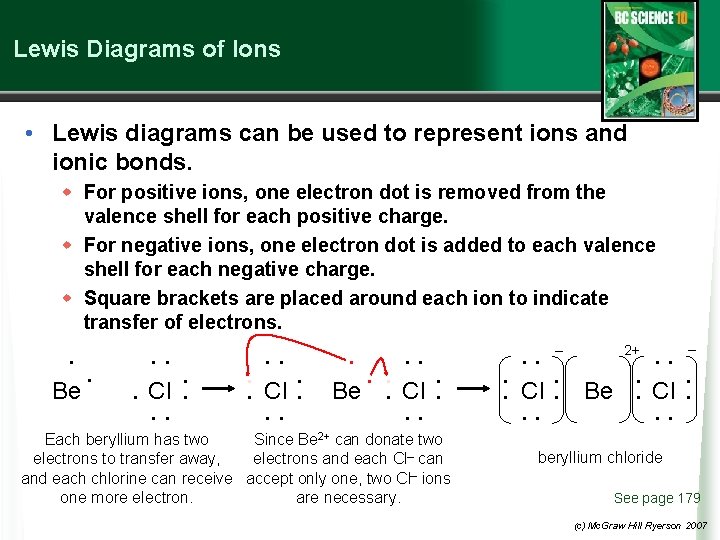
Lewis Diagrams of Ions • Lewis diagrams can be used to represent ions and ionic bonds. w For positive ions, one electron dot is removed from the valence shell for each positive charge. w For negative ions, one electron dot is added to each valence shell for each negative charge. w Square brackets are placed around each ion to indicate transfer of electrons. • • • Be • • • Cl • • • • • • Be • Cl • • • • Each beryllium has two Since Be 2+ can donate two electrons to transfer away, electrons and each Cl– can and each chlorine can receive accept only one, two Cl– ions one more electron. are necessary. • • Cl • • – • • • • Be • • 2+ • • Cl – • • • • beryllium chloride See page 179 (c) Mc. Graw Hill Ryerson 2007
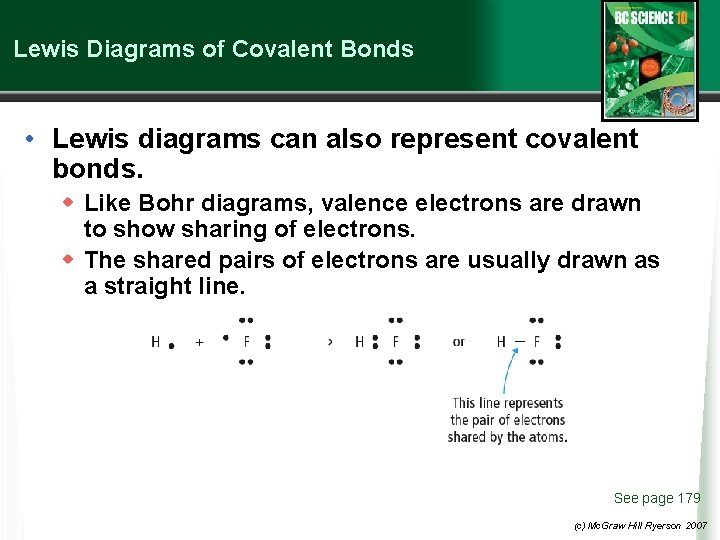
Lewis Diagrams of Covalent Bonds • Lewis diagrams can also represent covalent bonds. w Like Bohr diagrams, valence electrons are drawn to show sharing of electrons. w The shared pairs of electrons are usually drawn as a straight line. See page 179 (c) Mc. Graw Hill Ryerson 2007

Lewis Diagrams of Diatomic Molecules • Diatomic molecules, like O 2, are also easy to draw as Lewis diagrams. • • • • O • • • • O • • Several non-metals join to form diatomic molecules. • • O • • Valence electrons are shared, here in two pairs. Take the Section 4. 1 Quiz • • O • • O • • • • This is drawn as a double bond. See page 180 (c) Mc. Graw Hill Ryerson 2007
- Slides: 22