THE ATOMIC THEORY DEMOCRITUS 460 BC Greek philosopher

THE ATOMIC THEORY

DEMOCRITUS (460 BC) ØGreek philosopher proposes the existence of the atom ØHis Theory: § Are small hard particles. § Are made of single material formed into different shapes and sizes. § Are always moving, and they form different materials by joining together.

ARISTOTLE • He did not think there was a limit to the number of times matter could be divided. • He thought that all substances were built up from only four elements. Earth Water Fire Air

JOHN DALTON (1766 -1844) • British chemist • His Theory: • All substances are made of atoms that cannot be created, divided, or destroyed. • Atoms join with other atoms to make new substances. • Atoms of the same element are exactly alike, and atoms of different elements are different in mass and size.

• Dalton’s model of atom was accepted for about 100 years because he used it to support two fundamental law of nature – the law of conservation of mass and the law of definite proportion.

LAW OF CONSERVATION OF MASS (1766 -1844) • The law of conservation of mass states that the total mass present before a chemical reaction is the same as the total mass present after the chemical reaction; in other words, mass is conserved. The law of conservation of mass was formulated by Antoine Lavoisier (17431794) as a result of his combustion experiment, in which he observed that the mass of his original substance—a glass vessel, tin, and air— was equal to the mass of the produced substance—the glass vessel, “tin calyx”, and the remaining air.

LAW OF CONSTANT COMPOSITION • Joseph Proust (1754 -1826) formulated the law of constant composition (also called the law of definite proportions). This law states that if a compound is broken down into its constituent elements, the masses of the constituents will always have the same proportions, regardless of the quantity or source of the original substance. Joseph Proust based this law primarily on his experiments with basic copper carbonate.

LAW OF MULTIPLE PROPORTION • This states that if two elements (A and B) combine from different compounds, the different masses of one element (B) that combine with a fixed mass of another (A) can be express as a ratio of small whole number such as 1: 2, 2: 3, 3: 4, and so on.
J. J. THOMSON (1856 -1940) • English chemist and physicist; discovered 1 st subatomic particles. • His Theory: • Atoms contain negatively charged particles called electrons and positively charged matter. • Created a model to describe the atom as a sphere filled with positive matter with negative particles mixed in. • Referred to it as the plum pudding model.


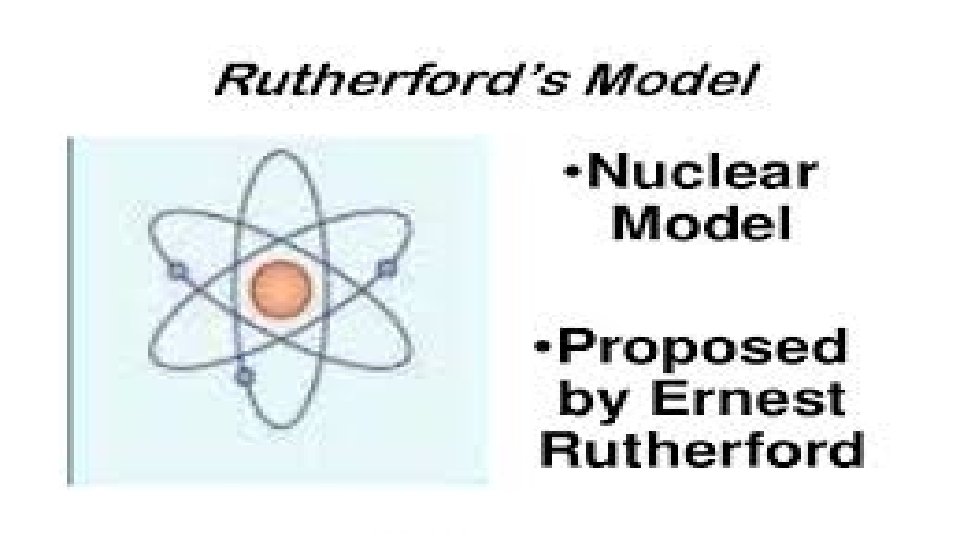
ERNEST RUTHERFORD (1871 -1937) • New Zealand physicist discovered the nucleus. • His Theory: • Small, dense, positively charged particle present in nucleus called a proton. • Electron travel around the nucleus, but their exact places cannot be described.

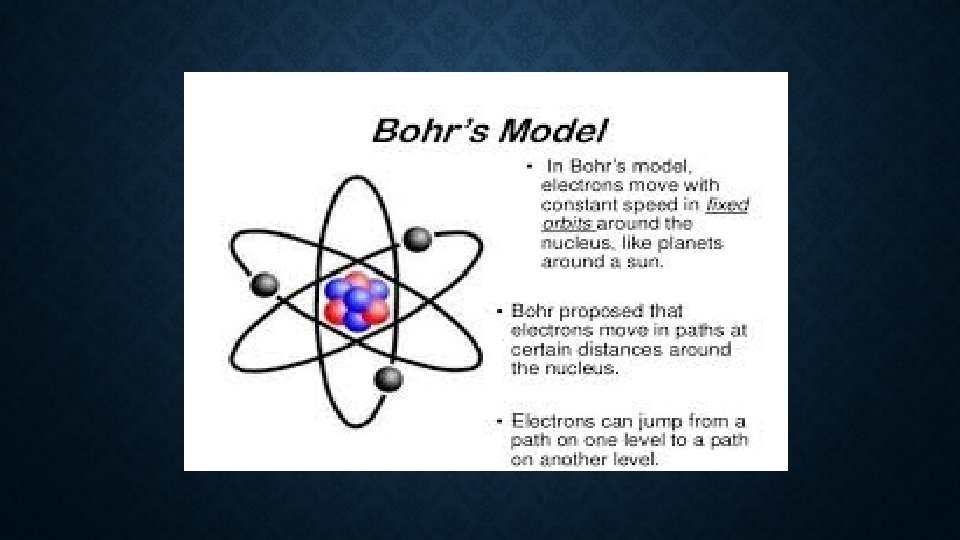
NEILS BOHR (1913) • Danish physicist; discovered energy levels. • His Theory: • Electrons travel around the nucleus in definite paths and fixed distances. • Electrons can jump from one level to a path in another level,

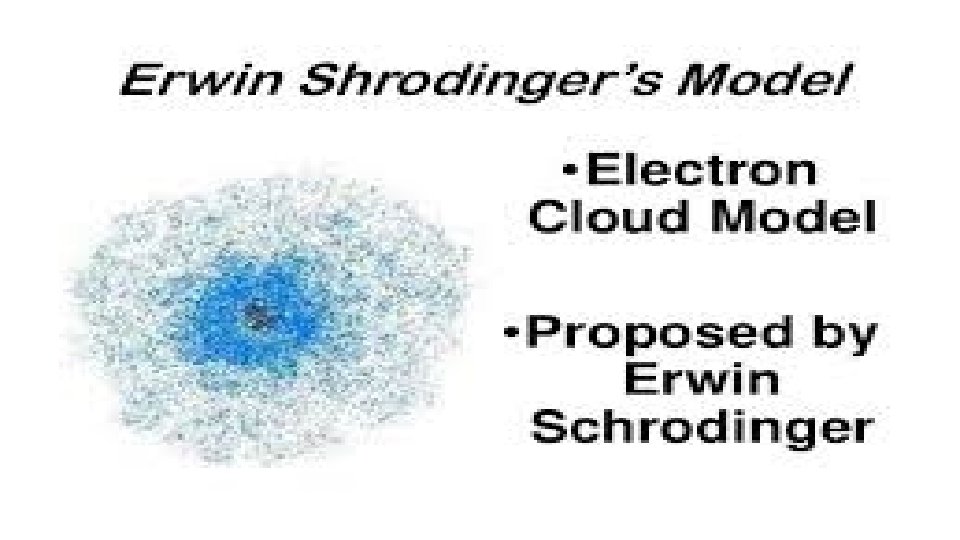
ERWIN SHCRODINGER (1924) • Autrian physicist; developed the electron cloud model. • His Theory: • The exact path of electrons cannot be predicted. • The region referred to as the electron cloud, is an area where electrons can likely be found.

JAMES CHADWICK (1932) • English physicist; discovered neutrons • His Theory: ØNeutrons have no electrical charge. ØNeutrons have a mass nearly equal to the mass of a proton.
MODERN THEORY OF THE ATOM • Atoms are composed of three main subatomic particles: the electron(-), proton(+), neutron. • Most of the mass of the atom is concentrated in the nucleus of the atom. • The protons and neutrons are located within the nucleus, while the electrons exist outside of the nucleus.

• In stable atoms, the number of protons is equal to the number of electrons. • The type of atom is determined by the number of protons it has. • The number of protons in an atom is equal to the atomic number. • The sum of the number of protons and neutrons in a particular atom is called the atomic mass.

• Valence electrons are the outermost electrons.
MODELS OF THE ATOM

• John Dalton imagined that the atom is a hard, indestructible sphere. • Joseph John Thomson proposed the “plum pudding model” of the atom (sometimes referred as raisin bread model). This tells that the negatively-charge particles are embedded in a positively-charge mass. • Ernest Rutherford proposed a “nuclear model” of the atom based on the findings of the gold foil experiment or alpha scattering experiment as he and his co-workers discovered the nucleus of the atom. The nucleus contains protons and neutrons.

• Niels Bohr proposed his “planetary model” of the atom which assumes that electrons move at certain permitted orbits (or definite energy levels) and are in allowed energy states. • The Quantum Mechanical Model is combine work of those scientists; Louie de Broglei, Erwin Schrodinger and Werner Karl Heisenberg. • De Broglei proposed that the electron can also be as wave. • Schrodinger used the idea to develop his wave equation to describe the Hydrogen atom • Heisenberg discovered the Uncertainty Principle stating that one cannot know exactly where an electron is, its energy or how it is moving.

Quantum numbers: 1. Principal Quantum Number (n)- this refers to the main energy level of an orbital and related to the total energy of the electron in the atom. 2. Azimuthal Quantum Number (l)- this also called angular momentum number or subsidiary number. It represents energy sublevels and can have values beginning with zero until the integers n-1 is reached.

• 3. Magnetic Quantum Number (m 1)- this describes the orientation of the orbital in the space and can have and integral value from -1 to +1, including zero. • 4. Spin Quantum Number (ms)- there are two orientations possible for electron spin: +1/2 and -1/2.
ISOTOPE, ATOMIC NUMBER, AND MASS NUMBER What are isotopes? ? ü Isotopes is just like twins, same genetic but different of DNA. üAtoms of the same element with same number of protons but different number of neutrons.
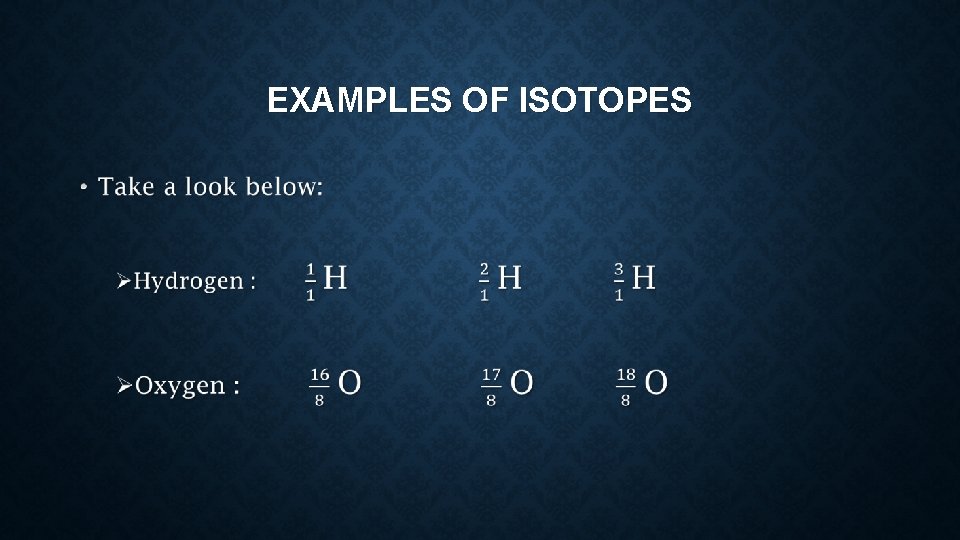
EXAMPLES OF ISOTOPES •
- Slides: 28