The Atom You think you know but do
The Atom You think you know, but do you? ? ?

Objectives • Compare and contrast the atomic models of Democritus and Dalton • Define an atom • Distinguish between the subatomic particles in terms of relative charge and mass • Describe the structure of the nuclear atom, including the locations of subatomic particles
What does the atom look like?
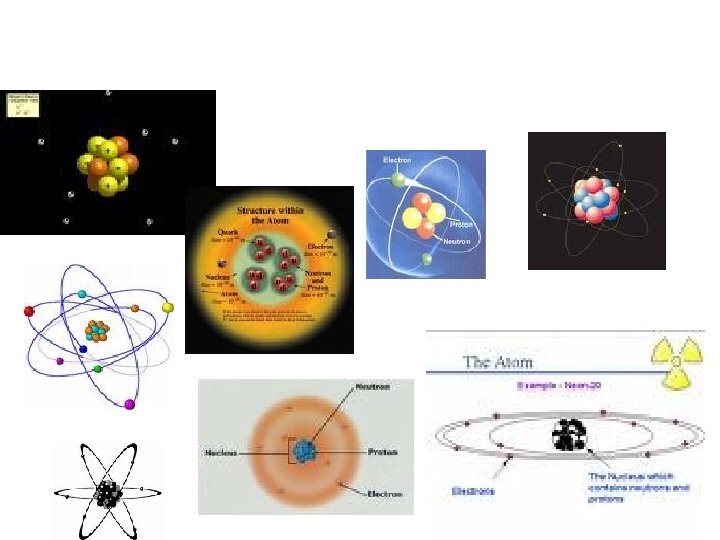
Atoms • Smallest particle of an element that retains the properties of that element • All matter is made of atoms

History of the Atom • Democritus, 400 B. C. – Greek philosopher – Universe is made of invisible units called atomos – Matter is predominantly empty space – Atoms differ in their size, shape and movement – Changes in matter result from changes in groupings of atoms, and not changes in the atoms themselves Atom is derived from the Greek word meaning “unable to be divided”

History of the Atom • Aristotle, 300 B. C. – Rejected Democritus’ Atomos Theory – Disagreed with the “Nothingness of empty space” in the Theory of Atomism – Hated Democritus so much ‘he wished all his books burnt’

History of the Atom • John Dalton, 1808, – English schoolteacher – Atomic Theory built on Democritus’ Theory of Atomism • Every element is made of tiny particles that cannot be subdivided (atoms) • Atoms of the same element are identical • Atoms cannot be created, divided, or destroyed • Atoms can combine in whole number ratios to form molecules • Chemical reactions are atoms being separated, combined, or rearranged

DALTON’S ATOM WOULD HAVE LOOKED LIKE THIS:
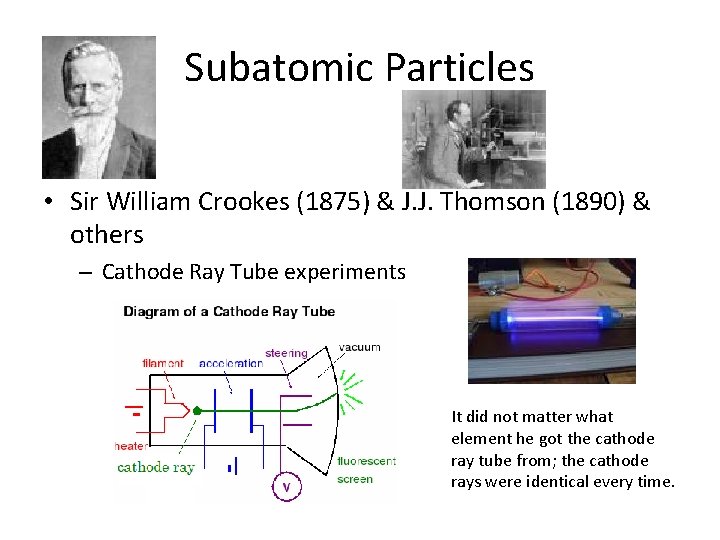
Subatomic Particles • Sir William Crookes (1875) & J. J. Thomson (1890) & others – Cathode Ray Tube experiments It did not matter what element he got the cathode ray tube from; the cathode rays were identical every time.

THOMSON’S ATOM WOULD HAVE LOOKED LIKE THIS:

Subatomic Particles - Electrons • Robert Millikan, American, 1909 • Oil Drop Experiment • Determined the charge (1. 59 E-19 Coulombs) and mass of electrons with amazing accuracy
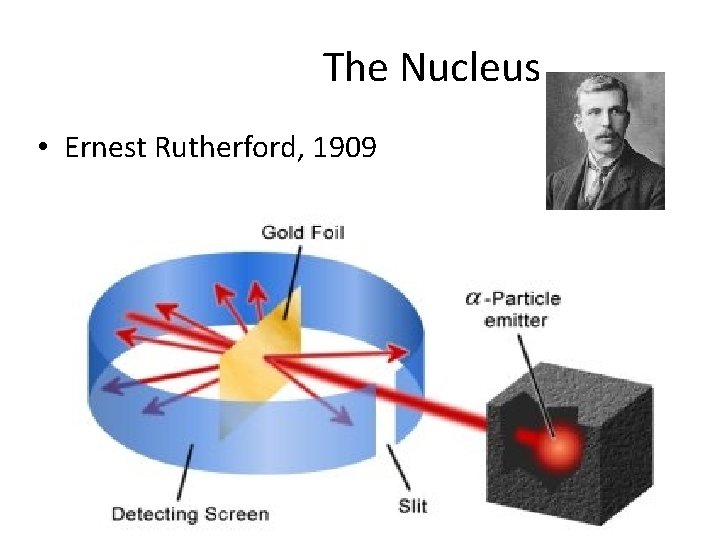
The Nucleus • Ernest Rutherford, 1909

RUTHERFORD’S ATOM WOULD HAVE LOOKED LIKE THIS:

Completing the Atom • James Chadwick, a student of Rutherford, 1932 • “extra mass” problem of isotopes…

CHADWICK’S ATOM WOULD HAVE LOOKED LIKE THIS: Why were these last particles so tough to detect? ? ?

Disclaimer….
- Slides: 17